Architectural design drives the biogeography of indoor bacterial communities - PubMed (original) (raw)
Architectural design drives the biogeography of indoor bacterial communities
Steven W Kembel et al. PLoS One. 2014.
Abstract
Background: Architectural design has the potential to influence the microbiology of the built environment, with implications for human health and well-being, but the impact of design on the microbial biogeography of buildings remains poorly understood. In this study we combined microbiological data with information on the function, form, and organization of spaces from a classroom and office building to understand how design choices influence the biogeography of the built environment microbiome.
Results: Sequencing of the bacterial 16S gene from dust samples revealed that indoor bacterial communities were extremely diverse, containing more than 32,750 OTUs (operational taxonomic units, 97% sequence similarity cutoff), but most communities were dominated by Proteobacteria, Firmicutes, and Deinococci. Architectural design characteristics related to space type, building arrangement, human use and movement, and ventilation source had a large influence on the structure of bacterial communities. Restrooms contained bacterial communities that were highly distinct from all other rooms, and spaces with high human occupant diversity and a high degree of connectedness to other spaces via ventilation or human movement contained a distinct set of bacterial taxa when compared to spaces with low occupant diversity and low connectedness. Within offices, the source of ventilation air had the greatest effect on bacterial community structure.
Conclusions: Our study indicates that humans have a guiding impact on the microbial biodiversity in buildings, both indirectly through the effects of architectural design on microbial community structure, and more directly through the effects of human occupancy and use patterns on the microbes found in different spaces and space types. The impact of design decisions in structuring the indoor microbiome offers the possibility to use ecological knowledge to shape our buildings in a way that will select for an indoor microbiome that promotes our health and well-being.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
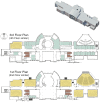
Figure 1. Architectural layout for two of four floors in Lillis Hall.
Restrooms (brown), offices (blue) and classrooms (yellow) are shown to illustrate space type distribution throughout Lillis. The first two floors of the building are primarily devoted to classrooms and share a similar floor-plan. The 3rd and 4th floors contain most offices in the building and also share a similar floor-plan. The building has a basement and penthouse spaces; these are largely building support spaces, including mechanical rooms and storage.
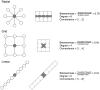
Figure 2. Network analysis metrics used to quantify spatial arrangement of spaces within Lillis Hall.
Examples in the left column follow classic network representation, while those in the right column embody the architectural translation of networks. Shaded nodes and building spaces correspond to centrality measures of betweenness (the number of shortest paths between all pairs of spaces that pass through a given space over the sum of all shortest paths between all pairs of spaces in the building) and degree (the number of connections a space has to other spaces); connectance distance (the number of doors between any two spaces) is a pairwise metric, shown here as the range of connectance distance values for each complete network/building. Since betweenness and degree strongly co-vary and are both measures of network centrality , they are considered together in some analyses.
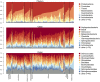
Figure 3. The taxonomic composition of bacterial communities sampled from dust in Lillis Hall.
Samples are organized by space type, and relative abundances are shown for groups comprising more than 1% (for phylum and class level) and 4% (for order level).
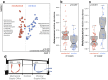
Figure 4. Dust communities within a building cluster by space type and are strongly correlated with building centrality and human occupancy.
Points represent centroids (±SE) from distance based redundancy analysis (DB-RDA). Space types hold significantly different communities (P = 0.005), though this is driven primarily by restrooms. Bacterial OTUs that have the strongest influence in sample dissimilarities are shown at the margins; numbers in parentheses indicate multiple OTUs in the same genus. Centrality (along y-axis) represents network betweenness and degree; human occupancy (along x-axis) represents annual occupied hours and human diversity. All four correlates (simple linear models as a factor of ordination axis) are significant along their respective axes (all P<0.001).
Figure 5. Offices contain significantly different dust microbial communities depending on ventilation source.
a) The first axis is constrained by whether or not offices have operable window louvers (blue) or not (red). Taxon names on either side are grouped from the 25 strongest weighting OTUs in either direction. b) Deinococcus were 1.7 times more abundant in mechanically ventilated offices compared to window ventilated offices. c) The opposite pattern was observed for Methylobacterium OTUs, which were 1.8 times more abundant in window ventilated offices. Boxplots delineate (from bottom) minimum, Q1, median, Q3, and maximum values; notches indicate 95% confidence intervals. d) Cross-sectional view of representative Lillis Hall offices. Offices on the south side of the building (left) received primarily mechanically ventilated air, while offices on the north side of the building (right) are equipped with operable windows as a primary ventilation air source.
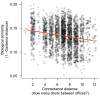
Figure 6. Offices in Lillis Hall show a strong distance-decay pattern.
When only considering a single space type, biological similarity (y-axis; 1 - Canberra distance) decreases with connectance distance (number of intermediate space boundaries [e.g., doors] one would walk through to travel the shortest distance between any two spaces) (Mantel test; R = 0.189; P = 0.002). The same pattern was also observed at the whole-building scale (not shown; Mantel test; R = 0.112; P = 0.001).
Similar articles
- Architectural design influences the diversity and structure of the built environment microbiome.
Kembel SW, Jones E, Kline J, Northcutt D, Stenson J, Womack AM, Bohannan BJ, Brown GZ, Green JL. Kembel SW, et al. ISME J. 2012 Aug;6(8):1469-79. doi: 10.1038/ismej.2011.211. Epub 2012 Jan 26. ISME J. 2012. PMID: 22278670 Free PMC article. - Diversity and seasonal dynamics of bacterial community in indoor environment.
Rintala H, Pitkäranta M, Toivola M, Paulin L, Nevalainen A. Rintala H, et al. BMC Microbiol. 2008 Apr 8;8:56. doi: 10.1186/1471-2180-8-56. BMC Microbiol. 2008. PMID: 18397514 Free PMC article. - Differences in bacterial composition between men's and women's restrooms and other common areas within a public building.
Dobbler PCT, Laureano ÁM, Sarzi DS, Cañón ERP, Metz GF, de Freitas AS, Takagaki BM, D Oliveira CB, Pylro VS, Copetti AC, Victoria F, Redmile-Gordon M, Morais DK, Roesch LFW. Dobbler PCT, et al. Antonie Van Leeuwenhoek. 2018 Apr;111(4):551-561. doi: 10.1007/s10482-017-0976-6. Epub 2017 Nov 10. Antonie Van Leeuwenhoek. 2018. PMID: 29127623 - House dust microbiome and human health risks.
Shan Y, Wu W, Fan W, Haahtela T, Zhang G. Shan Y, et al. Int Microbiol. 2019 Sep;22(3):297-304. doi: 10.1007/s10123-019-00057-5. Epub 2019 Jan 14. Int Microbiol. 2019. PMID: 30811000 Review. - Microbial, environmental and anthropogenic factors influencing the indoor microbiome of the built environment.
Rai S, Singh DK, Kumar A. Rai S, et al. J Basic Microbiol. 2021 Apr;61(4):267-292. doi: 10.1002/jobm.202000575. Epub 2021 Feb 1. J Basic Microbiol. 2021. PMID: 33522603 Review.
Cited by
- Evaluation of Aging Effect on the Durability of Antibacterial Treatments Applied on Textile Materials for the Automotive Industry.
Arese M, Mania I, Brunella V, Lambertini VG, Gorra R. Arese M, et al. ACS Omega. 2024 Jun 11;9(25):27169-27176. doi: 10.1021/acsomega.4c01272. eCollection 2024 Jun 25. ACS Omega. 2024. PMID: 38947847 Free PMC article. - Surveillance of Bacterial Load and Multidrug-Resistant Bacteria on Surfaces of Public Restrooms.
Ibrahim K, Tahsin M, Rahman A, Rahman SM, Rahman MM. Ibrahim K, et al. Int J Environ Res Public Health. 2024 Apr 30;21(5):574. doi: 10.3390/ijerph21050574. Int J Environ Res Public Health. 2024. PMID: 38791788 Free PMC article. - The potential importance of the built-environment microbiome and its impact on human health.
Bosch TCG, Wigley M, Colomina B, Bohannan B, Meggers F, Amato KR, Azad MB, Blaser MJ, Brown K, Dominguez-Bello MG, Ehrlich SD, Elinav E, Finlay BB, Geddie K, Geva-Zatorsky N, Giles-Vernick T, Gros P, Guillemin K, Haraoui LP, Johnson E, Keck F, Lorimer J, McFall-Ngai MJ, Nichter M, Pettersson S, Poinar H, Rees T, Tropini C, Undurraga EA, Zhao L, Melby MK. Bosch TCG, et al. Proc Natl Acad Sci U S A. 2024 May 14;121(20):e2313971121. doi: 10.1073/pnas.2313971121. Epub 2024 Apr 25. Proc Natl Acad Sci U S A. 2024. PMID: 38662573 Free PMC article. - Association between Residential Greenness and Human Microbiota: Evidence from Multiple Countries.
Zhang YD, Fan SJ, Zhang Z, Li JX, Liu XX, Hu LX, Knibbs LD, Dadvand P, Jalaludin B, Browning MHEM, Zhao T, Heinrich J, He Z, Chen CZ, Zhou Y, Dong GH, Yang BY. Zhang YD, et al. Environ Health Perspect. 2023 Aug;131(8):87010. doi: 10.1289/EHP12186. Epub 2023 Aug 16. Environ Health Perspect. 2023. PMID: 37585351 Free PMC article. - What Is Going on in Indoor Air Quality of a University Hospital in Northern Cyprus?
Güvenir M, Kaya U, Özçelik D, Süer K. Güvenir M, et al. Ethiop J Health Sci. 2023 May;33(3):541-546. doi: 10.4314/ejhs.v33i3.18. Ethiop J Health Sci. 2023. PMID: 37576165 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources