Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial - PubMed (original) (raw)
Randomized Controlled Trial
. 2014 Jun;82(3):448-459.
doi: 10.1037/a0035798. Epub 2014 Feb 3.
Affiliations
- PMID: 24491075
- PMCID: PMC4076008
- DOI: 10.1037/a0035798
Randomized Controlled Trial
Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial
Eric L Garland et al. J Consult Clin Psychol. 2014 Jun.
Abstract
Objective: Opioid pharmacotherapy is now the leading treatment for chronic pain, a problem that affects nearly one third of the U.S. population. Given the dramatic rise in prescription opioid misuse and opioid-related mortality, novel behavioral interventions are needed. The purpose of this study was to conduct an early-stage randomized controlled trial of Mindfulness-Oriented Recovery Enhancement (MORE), a multimodal intervention designed to simultaneously target mechanisms underpinning chronic pain and opioid misuse.
Method: Chronic pain patients (N = 115; mean age = 48 ± 14 years; 68% female) were randomized to 8 weeks of MORE or a support group (SG). Outcomes were measured at pre- and posttreatment, and at 3-month follow-up. The Brief Pain Inventory was used to assess changes in pain severity and interference. Changes in opioid use disorder status were measured by the Current Opioid Misuse Measure. Desire for opioids, stress, nonreactivity, reinterpretation of pain sensations, and reappraisal were also evaluated.
Results: MORE participants reported significantly greater reductions in pain severity (p = .038) and interference (p = .003) than SG participants, which were maintained by 3-month follow-up and mediated by increased nonreactivity and reinterpretation of pain sensations. Compared with SG participants, participants in MORE evidenced significantly less stress arousal (p = .034) and desire for opioids (p = .027), and were significantly more likely to no longer meet criteria for opioid use disorder immediately following treatment (p = .05); however, these effects were not sustained at follow-up.
Conclusions: Findings demonstrate preliminary feasibility and efficacy of MORE as a treatment for co-occurring prescription opioid misuse and chronic pain. (PsycINFO Database Record (c) 2014 APA, all rights reserved).
Figures
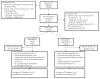
Figure 1
Flow diagram of the progress through the study.
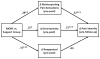
Figure 2
Multivariate path model of therapeutic mechanisms of Mindfulness-Oriented Recovery Enhancement on pain severity (N = 115). Model parameters reflect adjustment for the following covariates: age, gender, education, income level, and baseline opioid use disorder status.
Similar articles
- Mindfulness-oriented recovery enhancement reduces opioid misuse risk via analgesic and positive psychological mechanisms: A randomized controlled trial.
Garland EL, Hanley AW, Riquino MR, Reese SE, Baker AK, Salas K, Yack BP, Bedford CE, Bryan MA, Atchley R, Nakamura Y, Froeliger B, Howard MO. Garland EL, et al. J Consult Clin Psychol. 2019 Oct;87(10):927-940. doi: 10.1037/ccp0000390. J Consult Clin Psychol. 2019. PMID: 31556669 Free PMC article. Clinical Trial. - Mindfulness-Oriented Recovery Enhancement vs Supportive Group Therapy for Co-occurring Opioid Misuse and Chronic Pain in Primary Care: A Randomized Clinical Trial.
Garland EL, Hanley AW, Nakamura Y, Barrett JW, Baker AK, Reese SE, Riquino MR, Froeliger B, Donaldson GW. Garland EL, et al. JAMA Intern Med. 2022 Apr 1;182(4):407-417. doi: 10.1001/jamainternmed.2022.0033. JAMA Intern Med. 2022. PMID: 35226053 Free PMC article. Clinical Trial. - Mindfulness-Oriented Recovery Enhancement for Veterans and Military Personnel on Long-Term Opioid Therapy for Chronic Pain: A Randomized Clinical Trial.
Garland EL, Nakamura Y, Bryan CJ, Hanley AW, Parisi A, Froeliger B, Marchand WR, Donaldson GW. Garland EL, et al. Am J Psychiatry. 2024 Feb 1;181(2):125-134. doi: 10.1176/appi.ajp.20230272. Epub 2024 Jan 10. Am J Psychiatry. 2024. PMID: 38196335 Free PMC article. Clinical Trial. - Disrupting the downward spiral of chronic pain and opioid addiction with mindfulness-oriented recovery enhancement: a review of clinical outcomes and neurocognitive targets.
Garland EL. Garland EL. J Pain Palliat Care Pharmacother. 2014 Jun;28(2):122-9. doi: 10.3109/15360288.2014.911791. Epub 2014 May 20. J Pain Palliat Care Pharmacother. 2014. PMID: 24845547 Review. - Treating chronic pain: the need for non-opioid options.
Garland EL. Garland EL. Expert Rev Clin Pharmacol. 2014 Sep;7(5):545-50. doi: 10.1586/17512433.2014.928587. Epub 2014 Jun 11. Expert Rev Clin Pharmacol. 2014. PMID: 24918159 Review.
Cited by
- Virtual reality-based Mindfulness-Oriented Recovery Enhancement (MORE-VR) as an adjunct to medications for opioid use disorder: a Phase 1 trial.
Garland EL, Recasens M, Taple BJ, Donaldson GW, Weisberg RB. Garland EL, et al. Ann Med. 2024 Dec;56(1):2392870. doi: 10.1080/07853890.2024.2392870. Epub 2024 Aug 22. Ann Med. 2024. PMID: 39172534 Free PMC article. Clinical Trial. - Maternal psychophysiology profiles: associations with prenatal opioid use, maternal emotion dysregulation, and newborn neurobehavior.
Maylott SE, Reese SE, Zhou AM, Kaliush PR, Terrell S, Saenz C, Brown L, Crowell SE, Lester B, Smid MC, Shakib JH, Garland EL, Conradt E. Maylott SE, et al. Pediatr Res. 2024 Aug 3. doi: 10.1038/s41390-024-03399-7. Online ahead of print. Pediatr Res. 2024. PMID: 39097654 - PT-IN-MIND: study protocol for a multisite randomised feasibility trial investigating physical therapy with integrated mindfulness (PT-IN-MIND) for patients with chronic musculoskeletal pain and long-term opioid treatment who attend outpatient physical therapy.
Magel JS, Beneciuk JM, Siantz E, Fritz J, Garland EL, Hanley A, Shen J, Blosser P, Matev T, Gordon AJ. Magel JS, et al. BMJ Open. 2024 Jul 30;14(7):e082611. doi: 10.1136/bmjopen-2023-082611. BMJ Open. 2024. PMID: 39079926 Free PMC article. - Feasibility of Web-Based Single-Session Empowered Relief in Patients With Chronic Pain Taking Methadone or Buprenorphine: Protocol for a Single-Arm Trial.
Klein MR, Darnall BD, You DS. Klein MR, et al. JMIR Res Protoc. 2024 Jun 6;13:e53784. doi: 10.2196/53784. JMIR Res Protoc. 2024. PMID: 38843513 Free PMC article. - Enhancing Chronic Non-Cancer Pain Management: A Systematic Review of Mindfulness Therapies and Guided Imagery Interventions.
Pinto BM, Tavares I, Pozza DH. Pinto BM, et al. Medicina (Kaunas). 2024 Apr 23;60(5):686. doi: 10.3390/medicina60050686. Medicina (Kaunas). 2024. PMID: 38792869 Free PMC article. Review.
References
- Alcaro A, Panksepp J. The SEEKING mind: Primal neuro-affective substrates for appetitive incentive states and their pathological dynamics in addictions and depression. Neuroscience & Biobehavioral Reviews. 2011;35(9):1805–1820. - PubMed
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27–45. - PubMed
- Borkovec TD, Nau SD. Credibility of analogue therapy rationales. Journal of Behavior Therapy and Experimental Psychiatry. 1972;3(4):257–260.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical