Endothelial nitric oxide synthase in red blood cells: key to a new erythrocrine function? - PubMed (original) (raw)
Review
Endothelial nitric oxide synthase in red blood cells: key to a new erythrocrine function?
Miriam M Cortese-Krott et al. Redox Biol. 2014.
Abstract
Red blood cells (RBC) have been considered almost exclusively as a transporter of metabolic gases and nutrients for the tissues. It is an accepted dogma that RBCs take up and inactivate endothelium-derived NO via rapid reaction with oxyhemoglobin to form methemoglobin and nitrate, thereby limiting NO available for vasodilatation. Yet it has also been shown that RBCs not only act as "NO sinks", but exert an erythrocrine function - i.e an endocrine function of RBC - by synthesizing, transporting and releasing NO metabolic products and ATP, thereby potentially controlling systemic NO bioavailability and vascular tone. Recent work from our and others laboratory demonstrated that human RBCs carry an active type 3, endothelial NO synthase (eNOS), constitutively producing NO under normoxic conditions, the activity of which is compromised in patients with coronary artery disease. In this review we aim to discuss the potential role of red cell eNOS in RBC signaling and function, and to critically revise evidence to this date showing a role of non-endothelial circulating eNOS in cardiovascular pathophysiology.
Keywords: Cardiovascular disease; Nitric oxide; Red blood cells; eNOS.
Figures
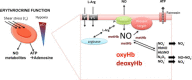
Graphical abstract
Fig. 1
Upper panel: The expression of eNOS in the endothelium of the vessels decreases along the vascular tree, while blood cell eNOS carried by all main blood populations is equally distributed. Middle panel: In the blood compartment a circulating pool of NO metabolites is formed and transported in plasma and RBC. Bottom panel: NO can be synthesized enzymatically from L-arginine under normoxic conditions in a reaction catalyzed by a NOS, and under hypoxic conditions from nitrite by the nitrate reductase activity of proteins including deoxyhemoglobin (deoxyHb), deoxymyoglobin (deoxyMb), xantine oxidoreductase (XOR), carbonic anydrase (CA), and cytochrome C (cytC)/cytC-oxidase. eNOS, endothelial nitric oxide synthase; LNO2, nitrated lipids; RSNO, nitrosothiols; RNNO, nitrosamines.
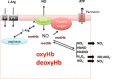
Fig. 2
Potential role of red cell eNOS in signalling. L-arginine is imported into the RBC via the cationic amino acid transporters y+/CAT and/or y+L. Both localization of eNOS on the cytoplasmic side of the RBC membrane and protein-protein interactions may play a central role in effectively separating eNOS from arginase, as well as NO production from NO scavenging by oxyhemoglobin (oxyHb). Local formation of methemoglobin (metHb) by reaction of eNOS-derived NO may protect further produced NO from degradation by oxyHb, allowing NO to interact with target proteins in the immediate vicinity of eNOS to exert intracellular signaling function, leading to activation of NO signaling pathways. An example of such a target might be the Pannexin-1 channel.
Fig. 3
Creation in vivo chimera models for studying the role of blood cell eNOS. To analyze the role of blood cell eNOS we created chimeras carrying eNOS in the blood only (group II) or lacking eNOS in the blood only (group III) and respective controls (groups I, IV) by cross-transplanting the bone marrow of wild type (WT) or eNOS knockout (eNOS-/-) mice into lethal irradiated mice. We found that also blood cell eNOS play a role in control of vascular tone and cardioprotection.
Fig. 4
Pathophysiological significance of red cell eNOS. An accumulating body of evidence identifies RBCs as active contributors to vascular homeostasis and cardioprotection. These effects might be due to the ability of RBCs to secrete vasoactive and cardioprotective molecules, a property which we here define as erythrocrine function, and might be dependent on the activity of red cell eNOS. Further studies are required to unravel its nature, regulation and pathophysiological significance.
Comment in
- Can nitric oxide synthase activity be unequivocally measured in red Blood cells and platelets? if yes, by which assay?
Tsikas D. Tsikas D. Redox Biol. 2015 Aug;5:409-11. doi: 10.1016/j.redox.2015.08.003. Redox Biol. 2015. PMID: 27135111 No abstract available. - Response to commentary.
Cortese-Krott MM. Cortese-Krott MM. Redox Biol. 2015 Aug;5:411-2. doi: 10.1016/j.redox.2015.08.004. Redox Biol. 2015. PMID: 27135112 No abstract available.
Similar articles
- Red Blood Cell and Endothelial eNOS Independently Regulate Circulating Nitric Oxide Metabolites and Blood Pressure.
Leo F, Suvorava T, Heuser SK, Li J, LoBue A, Barbarino F, Piragine E, Schneckmann R, Hutzler B, Good ME, Fernandez BO, Vornholz L, Rogers S, Doctor A, Grandoch M, Stegbauer J, Weitzberg E, Feelisch M, Lundberg JO, Isakson BE, Kelm M, Cortese-Krott MM. Leo F, et al. Circulation. 2021 Sep 14;144(11):870-889. doi: 10.1161/CIRCULATIONAHA.120.049606. Epub 2021 Jul 7. Circulation. 2021. PMID: 34229449 Free PMC article. - Human red blood cells at work: identification and visualization of erythrocytic eNOS activity in health and disease.
Cortese-Krott MM, Rodriguez-Mateos A, Sansone R, Kuhnle GG, Thasian-Sivarajah S, Krenz T, Horn P, Krisp C, Wolters D, Heiß C, Kröncke KD, Hogg N, Feelisch M, Kelm M. Cortese-Krott MM, et al. Blood. 2012 Nov 15;120(20):4229-37. doi: 10.1182/blood-2012-07-442277. Epub 2012 Sep 24. Blood. 2012. PMID: 23007404 - Red blood cell endothelial nitric oxide synthase does not modulate red blood cell storage hemolysis.
Kanias T, Wang L, Lippert A, Kim-Shapiro DB, Gladwin MT. Kanias T, et al. Transfusion. 2013 May;53(5):981-9. doi: 10.1111/j.1537-2995.2012.03850.x. Epub 2012 Aug 15. Transfusion. 2013. PMID: 22897637 Free PMC article. - Endothelial nitric oxide synthase in the microcirculation.
Shu X, Keller TC 4th, Begandt D, Butcher JT, Biwer L, Keller AS, Columbus L, Isakson BE. Shu X, et al. Cell Mol Life Sci. 2015 Dec;72(23):4561-75. doi: 10.1007/s00018-015-2021-0. Epub 2015 Aug 25. Cell Mol Life Sci. 2015. PMID: 26390975 Free PMC article. Review. - Exercise-Induced Cardioprotection via eNOS: A Putative Role of Red Blood Cell Signaling.
Suvorava T, Cortese-Krott MM. Suvorava T, et al. Curr Med Chem. 2018;25(34):4457-4474. doi: 10.2174/0929867325666180307112557. Curr Med Chem. 2018. PMID: 29521199 Review.
Cited by
- Oxidative Stress and Arginine/Nitric Oxide Pathway in Red Blood Cells Derived from Patients with Prediabetes.
Eligini S, Porro B, Werba JP, Capra N, Genovese S, Greco A, Cavalca V, Banfi C. Eligini S, et al. Biomedicines. 2022 Jun 14;10(6):1407. doi: 10.3390/biomedicines10061407. Biomedicines. 2022. PMID: 35740426 Free PMC article. - Induction of Nitric-Oxide Metabolism in Enterocytes Alleviates Colitis and Inflammation-Associated Colon Cancer.
Stettner N, Rosen C, Bernshtein B, Gur-Cohen S, Frug J, Silberman A, Sarver A, Carmel-Neiderman NN, Eilam R, Biton I, Pevsner-Fischer M, Zmora N, Brandis A, Bahar Halpern K, Mazkereth R, di Bernardo D, Brunetti-Pierri N, Premkumar MH, Dank G, Nagamani SCS, Jung S, Harmelin A, Erez A. Stettner N, et al. Cell Rep. 2018 May 15;23(7):1962-1976. doi: 10.1016/j.celrep.2018.04.053. Cell Rep. 2018. PMID: 29768197 Free PMC article. - Modulation of Local and Systemic Heterocellular Communication by Mechanical Forces: A Role of Endothelial Nitric Oxide Synthase.
Erkens R, Suvorava T, Kramer CM, Diederich LD, Kelm M, Cortese-Krott MM. Erkens R, et al. Antioxid Redox Signal. 2017 Jun 1;26(16):917-935. doi: 10.1089/ars.2016.6904. Epub 2017 Feb 16. Antioxid Redox Signal. 2017. PMID: 27927026 Free PMC article. Review. - Human erythrocytes, nuclear factor kappaB (NFκB) and hydrogen sulfide (H2S) - from non-genomic to genomic research.
Ghashghaeinia M, Mrowietz U. Ghashghaeinia M, et al. Cell Cycle. 2021 Oct;20(20):2091-2101. doi: 10.1080/15384101.2021.1972557. Epub 2021 Sep 24. Cell Cycle. 2021. PMID: 34559024 Free PMC article. - Biological Assessment of the NO-Dependent Endothelial Function.
Boughaleb H, Lobysheva I, Dei Zotti F, Balligand JL, Montiel V. Boughaleb H, et al. Molecules. 2022 Nov 16;27(22):7921. doi: 10.3390/molecules27227921. Molecules. 2022. PMID: 36432022 Free PMC article. Review.
References
- Moncada S., Palmer R.M.J., Higgs E.A. Nitric oxide, biology pathophysiology and pharmacology. Pharmacol. Rev. 1991;43:109–142. - PubMed
- Furchgott R.F., Zawadzki J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. - PubMed
- Ignarro L.J. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ. Res. 1989;65:1–21. - PubMed
- Palmer R.M., Ferrige A.G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. - PubMed
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;2:997–1000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources