Role of the intestinal microbiota in resistance to colonization by Clostridium difficile - PubMed (original) (raw)
Review
Role of the intestinal microbiota in resistance to colonization by Clostridium difficile
Robert A Britton et al. Gastroenterology. 2014 May.
Abstract
Antibiotic-associated infection with the bacterial pathogen Clostridium difficile is a major cause of morbidity and increased health care costs. C difficile infection follows disruption of the indigenous gut microbiota by antibiotics. Antibiotics create an environment within the intestine that promotes C difficile spore germination, vegetative growth, and toxin production, leading to epithelial damage and colitis. Studies of patients with C difficile infection and animal models have shown that the indigenous microbiota can inhibit expansion and persistence of C difficile. Although the specific mechanisms of these processes are not known, they are likely to interfere with key aspects of the pathogen's physiology, including spore germination and competitive growth. Increasing our understanding of how the intestinal microbiota manage C difficile could lead to better means of controlling this important nosocomial pathogen.
Keywords: Antibiotics; C difficile; Colonization Resistance; Microbial Ecology; Microbiome.
Copyright © 2014 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest: VBY is a recipient of an ASPIRE research award.
Figures
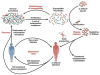
Figure 1. Cycle of CDI
Antibiotic administration alters the indigenous intestinal microbiota, producing an environment that permits germination of C difficile spores and expansion of the pathogen. C difficile produces toxins that cause colitis and other symptoms. Antibiotics directed against C difficile can decrease the load of the pathogen and toxin production. Returning the microbiota to a state of colonization resistance cures CDI. However, if the microbiota is unable to restore resistance to colonization by C difficile patients have recurring CDI. In certain cases, repeat courses of anti-C difficile antibiotic therapy can eradicate the pathogen. In other cases, therapeutic restoration of a diverse microbiota via FMT is required to overcome CDI.
Figure 2. Bile Acids Affect the Ability of C difficile to Colonize the Intestinal Tract
In the healthy microbiota, levels of cholate (CA) and chenodeoxycholate (CDCA) are high in the small intestine, and chenodeoxycholate inhibits germination. Because cholate is less well absorbed than chenodeoxycholate in the terminal ileum, the net effect is an increased ratio of cholate: chenodeoxycholate, resulting in increased spore germination –. As vegetative C difficile cells move into the colon, they are exposed to the toxic effects of deoxycholate (DCA), further preventing C difficile from gaining a foothold in the colon. When the microbiota is disrupted with antibiotics bacterial transformation of primary bile acids (such as CA to DCA) can be greatly suppressed. In this case CA levels remain high while bacterial transformation of CA to DCA does not occur. This results in increased CA mediated spore germination and a loss of inhibitory effects of deoxycholate on C difficile growth. The net effect is the expansion of this pathogen will readily occur in the colon.
Similar articles
- Recurrent Clostridium difficile infections: the importance of the intestinal microbiota.
Zanella Terrier MC, Simonet ML, Bichard P, Frossard JL. Zanella Terrier MC, et al. World J Gastroenterol. 2014 Jun 21;20(23):7416-23. doi: 10.3748/wjg.v20.i23.7416. World J Gastroenterol. 2014. PMID: 24966611 Free PMC article. Review. - Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile.
Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, Littmann E, van den Brink MR, Jenq RR, Taur Y, Sander C, Cross JR, Toussaint NC, Xavier JB, Pamer EG. Buffie CG, et al. Nature. 2015 Jan 8;517(7533):205-8. doi: 10.1038/nature13828. Epub 2014 Oct 22. Nature. 2015. PMID: 25337874 Free PMC article. - Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection.
Weingarden AR, Chen C, Bobr A, Yao D, Lu Y, Nelson VM, Sadowsky MJ, Khoruts A. Weingarden AR, et al. Am J Physiol Gastrointest Liver Physiol. 2014 Feb 15;306(4):G310-9. doi: 10.1152/ajpgi.00282.2013. Epub 2013 Nov 27. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24284963 Free PMC article. - From stool transplants to next-generation microbiota therapeutics.
Petrof EO, Khoruts A. Petrof EO, et al. Gastroenterology. 2014 May;146(6):1573-1582. doi: 10.1053/j.gastro.2014.01.004. Epub 2014 Jan 8. Gastroenterology. 2014. PMID: 24412527 Free PMC article. Review. - Bile salt metabolism is not the only factor contributing to Clostridioides (Clostridium) difficile disease severity in the murine model of disease.
Jukes CA, Ijaz UZ, Buckley A, Spencer J, Irvine J, Candlish D, Li JV, Marchesi JR, Douce G. Jukes CA, et al. Gut Microbes. 2020 May 3;11(3):481-496. doi: 10.1080/19490976.2019.1678996. Epub 2019 Dec 2. Gut Microbes. 2020. PMID: 31793403 Free PMC article.
Cited by
- Probiotic Gastrointestinal Transit and Colonization After Oral Administration: A Long Journey.
Han S, Lu Y, Xie J, Fei Y, Zheng G, Wang Z, Liu J, Lv L, Ling Z, Berglund B, Yao M, Li L. Han S, et al. Front Cell Infect Microbiol. 2021 Mar 10;11:609722. doi: 10.3389/fcimb.2021.609722. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33791234 Free PMC article. Review. - Living fabrication of functional semi-interpenetrating polymeric materials.
Dai Z, Yang X, Wu F, Wang L, Xiang K, Li P, Lv Q, Tang J, Dohlman A, Dai L, Shen X, You L. Dai Z, et al. Nat Commun. 2021 Jun 8;12(1):3422. doi: 10.1038/s41467-021-23812-7. Nat Commun. 2021. PMID: 34103521 Free PMC article. - A multicenter, randomized controlled comparison of three renutrition strategies for the management of moderate acute malnutrition among children aged from 6 to 24 months (the MALINEA project).
Vray M, Hedible BG, Adam P, Tondeur L, Manirazika A, Randremanana R, Mainassara H, Briend A, Artaud C, von Platen C, Altmann M, Jambou R. Vray M, et al. Trials. 2018 Dec 4;19(1):666. doi: 10.1186/s13063-018-3027-3. Trials. 2018. PMID: 30514364 Free PMC article. - The Phylogeny and Biological Function of Gastric Juice-Microbiological Consequences of Removing Gastric Acid.
Martinsen TC, Fossmark R, Waldum HL. Martinsen TC, et al. Int J Mol Sci. 2019 Nov 29;20(23):6031. doi: 10.3390/ijms20236031. Int J Mol Sci. 2019. PMID: 31795477 Free PMC article. Review. - Defining Dysbiosis in Patients with Urolithiasis.
Zampini A, Nguyen AH, Rose E, Monga M, Miller AW. Zampini A, et al. Sci Rep. 2019 Apr 1;9(1):5425. doi: 10.1038/s41598-019-41977-6. Sci Rep. 2019. PMID: 30932002 Free PMC article.
References
- Hall IC, O’Toole E. Intestinal flora in new-born infants with the description of a new pathogic anaerobe, Bacillus difficilis. Am J Dis Child. 1935;49:390–402.
- Thompson CM, Jr, Gilligan PH, Fisher MC, et al. Clostridium difficile cytotoxin in a pediatric population. Am J Dis Child. 1983;137:271–4. - PubMed
- Rousseau C, Poilane I, De Pontual L, et al. Clostridium difficile carriage in healthy infants in the community: a potential reservoir for pathogenic strains. Clin Infect Dis. 2012;55:1209–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19AI090871/AI/NIAID NIH HHS/United States
- U19 AI090871/AI/NIAID NIH HHS/United States
- U19 AI090872/AI/NIAID NIH HHS/United States
- P30 DK034933/DK/NIDDK NIH HHS/United States
- U19090872/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical