PAK signalling during the development and progression of cancer - PubMed (original) (raw)
Review
PAK signalling during the development and progression of cancer
Maria Radu et al. Nat Rev Cancer. 2014 Jan.
Abstract
p21-Activated kinases (PAKs) are positioned at the nexus of several oncogenic signalling pathways. Overexpression or mutational activation of PAK isoforms frequently occurs in various human tumours, and recent data suggest that excessive PAK activity drives many of the cellular processes that are the hallmarks of cancer. In this Review, we discuss the mechanisms of PAK activation in cancer, the key substrates that mediate the developmental and oncogenic effects of this family of kinases, and how small-molecule inhibitors of these enzymes might be best developed and deployed for the treatment of cancer.
Figures
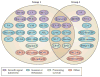
Figure 1. Validated Pak substrates and their roles in the hallmarks of cancer
Substrates of group I and II Paks are listed according to their putative role in oncogenic signaling. In some cases (e.g., c-Raf), given substrates play roles in multiple cellular functions, but may be listed only once to avoid visual clutter. Pak substrates are included only if reported by more than one group or if the reported site of phosphorylation is represented in the PhosphoSitePlus database (
http://www.phosphosite.org/homeAction.do
). Abbreviations: CALD1, caldesmin 1; ER, estrogen receptor; FlnA, Filamin A; H3, histone 3; LIMK, LIM kinase; MLC, myosin light chain; MLCK, myosin light chain kinase; Plk1, polo-kinase-1; SSH, Slingshot; Stmn1, stathmin-1; Vim, Vimentin.
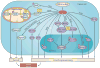
Figure 2. Role of Pak in growth signal autonomy and cell survival
Group I Paks are activated by both Cdc42 and Rac, whereas Group II Paks are activated only by Cdc42. Selected Pak substrates are depicted according to their role in oncogenic signaling. Phosphorylation sites, where known, are listed for each substrate. Dashed lines indicate that Pak has been implicated in activation of the substrate, but that the mechanism is uncertain.
Figure 3. Pak signaling in angiogenesis and modulation of vascular permeability
Paks control critical cellular events required for angiogenesis, including endothelial cell proliferation, survival, attachment and migration. In endothelial cells, the phosphorylation of BAD and RAF1 by Pak protects against apoptotic stimuli by promoting RAF1 translocation to mitochondria and the displacement of BAD/BCL2 complexes. As seen in other cellular contexts, in endothelial cells, the Erk pathway regulates cellular proliferation and migration when initiated by activation of Rac/Pak pathway. The control of vascular permeability by Pak is mediated by modulation of cellular contractility and cell:cell adhesion molecules. In one model, direct phosphorylation of myosin light chain (MLC) by Pak leads to increased contractility and increased endothelial permeability, as has been seen in certain experimental settings. Pak has also been proposed to disrupt endothelial cell:cell junctions by direct phosphorylation and subsequent internalization and degradation of VE cadherin. In another model, activated Pak phosphorylates and inhibits GEF-H1 (also known as ARHGEF2), leading to diminished RHOA/RHO-associated coiled-coil containing protein kinase (ROCK)/MLC activity, decreased contractility and decreased endothelial permeability, This model is consistent with data showing that Pak protects against an increase in permeability in a hypoxia induced pulmonary hypertension model and in a Pak2a knockout zebrafish model (dotted lines).
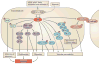
Figure 4. Specificity of Pak inhibitors
The human kinome is represented on a radar plot. The Pak family is oriented to 12 o’clock, emphasized by a red marking. The length of gray areas emanating from the bulls-eye represents the degree of kinase inhibition by each inhibitor shown below. Kinase families are indicated by different colors, as shown in the key to the right of the diagram: TK – Tyrosine kinase; TKL – Tyrosine kinase-like; STE – Homologs of yeast Sterile 20 kinases; CK1 – Casein kinase 1; AGC –Containing PKA, PKG, PKC families; CAMK – Calcium/calmodulin-dependent protein kinase; CMGC –Containing CDK, MAPK, GSK3, CLK families; ATYPICAL – Atypical protein kinase. Primary specificity data are derived from References , , , and .
Similar articles
- Role of p-21-activated kinases in cancer progression.
King H, Nicholas NS, Wells CM. King H, et al. Int Rev Cell Mol Biol. 2014;309:347-87. doi: 10.1016/B978-0-12-800255-1.00007-7. Int Rev Cell Mol Biol. 2014. PMID: 24529727 Review. - P21 activated kinase signaling in cancer.
Rane CK, Minden A. Rane CK, et al. Semin Cancer Biol. 2019 Feb;54:40-49. doi: 10.1016/j.semcancer.2018.01.006. Epub 2018 Jan 9. Semin Cancer Biol. 2019. PMID: 29330094 Review. - PAK2 as a therapeutic target in cancer: Mechanisms, challenges, and future perspectives.
Chen XP, Yang ZT, Yang SX, Li EM, Xie L. Chen XP, et al. Biochim Biophys Acta Rev Cancer. 2025 Feb;1880(1):189246. doi: 10.1016/j.bbcan.2024.189246. Epub 2024 Dec 16. Biochim Biophys Acta Rev Cancer. 2025. PMID: 39694422 Review. - The Role of the p21-Activated Kinase Family in Tumor Immunity.
Lu T, Huo Z, Zhang Y, Li X. Lu T, et al. Int J Mol Sci. 2025 Apr 20;26(8):3885. doi: 10.3390/ijms26083885. Int J Mol Sci. 2025. PMID: 40332759 Free PMC article. Review. - Therapeutic Potential of Targeting PAK Signaling.
Senapedis W, Crochiere M, Baloglu E, Landesman Y. Senapedis W, et al. Anticancer Agents Med Chem. 2016;16(1):75-88. doi: 10.2174/1871520615666150617111414. Anticancer Agents Med Chem. 2016. PMID: 26081410 Review.
Cited by
- Overexpression of P21-activated kinase 4 is associated with poor prognosis in non-small cell lung cancer and promotes migration and invasion.
Cai S, Ye Z, Wang X, Pan Y, Weng Y, Lao S, Wei H, Li L. Cai S, et al. J Exp Clin Cancer Res. 2015 May 15;34(1):48. doi: 10.1186/s13046-015-0165-2. J Exp Clin Cancer Res. 2015. PMID: 25975262 Free PMC article. - Effects of p21-activated kinase 1 inhibition on 11q13-amplified ovarian cancer cells.
Prudnikova TY, Villamar-Cruz O, Rawat SJ, Cai KQ, Chernoff J. Prudnikova TY, et al. Oncogene. 2016 Apr 28;35(17):2178-85. doi: 10.1038/onc.2015.278. Epub 2015 Aug 10. Oncogene. 2016. PMID: 26257058 Free PMC article. - PAK4-NAMPT Dual Inhibition as a Novel Strategy for Therapy Resistant Pancreatic Neuroendocrine Tumors.
Mpilla G, Aboukameel A, Muqbil I, Kim S, Beydoun R, Philip PA, Mohammad RM, Kamgar M, Shidham V, Senapedis W, Baloglu E, Li J, Dyson G, Xue Y, El-Rayes B, Azmi AS. Mpilla G, et al. Cancers (Basel). 2019 Nov 29;11(12):1902. doi: 10.3390/cancers11121902. Cancers (Basel). 2019. PMID: 31795447 Free PMC article. - Mislocalized cytoplasmic p27 activates PAK1-mediated metastasis and is a prognostic factor in osteosarcoma.
Chen X, Cates JMM, Du YC, Jain A, Jung SY, Li XN, Hicks JM, Man TK. Chen X, et al. Mol Oncol. 2020 Apr;14(4):846-864. doi: 10.1002/1878-0261.12624. Epub 2020 Feb 14. Mol Oncol. 2020. PMID: 31872963 Free PMC article. - Subtype-specific kinase dependency regulates growth and metastasis of poor-prognosis mesenchymal colorectal cancer.
Buikhuisen JY, Gomez Barila PM, Cameron K, Suijkerbuijk SJE, Lieftink C, di Franco S, Krotenberg Garcia A, Uceda Castro R, Lenos KJ, Nijman LE, Torang A, Longobardi C, de Jong JH, Dekker D, Stassi G, Vermeulen L, Beijersbergen RL, van Rheenen J, Huveneers S, Medema JP. Buikhuisen JY, et al. J Exp Clin Cancer Res. 2023 Mar 3;42(1):56. doi: 10.1186/s13046-023-02600-9. J Exp Clin Cancer Res. 2023. PMID: 36869386 Free PMC article.
References
- Dart AE, Wells CM. P21-activated kinase 4--not just one of the PAK. European journal of cell biology. 2013;92:129–38. - PubMed
- Parsons DW, et al. Colorectal cancer: mutations in a signalling pathway. Nature. 2005;436:792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA142928/CA/NCI NIH HHS/United States
- R01 CA058836/CA/NCI NIH HHS/United States
- R01 CA148805/CA/NCI NIH HHS/United States
- P30 CA006927/CA/NCI NIH HHS/United States
- T32 CA009035/CA/NCI NIH HHS/United States
- R01 CA098830/CA/NCI NIH HHS/United States
- R01 CA58836/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources