TGF-β: duality of function between tumor prevention and carcinogenesis - PubMed (original) (raw)
Review
TGF-β: duality of function between tumor prevention and carcinogenesis
Daniel R Principe et al. J Natl Cancer Inst. 2014 Feb.
Abstract
Several mechanisms underlying tumor progression have remained elusive, particularly in relation to transforming growth factor beta (TGF-β). Although TGF-β initially inhibits epithelial growth, it appears to promote the progression of advanced tumors. Defects in normal TGF-β pathways partially explain this paradox, which can lead to a cascade of downstream events that drive multiple oncogenic pathways, manifesting as several key features of tumorigenesis (uncontrolled proliferation, loss of apoptosis, epithelial-to-mesenchymal transition, sustained angiogenesis, evasion of immune surveillance, and metastasis). Understanding the mechanisms of TGF-β dysregulation will likely reveal novel points of convergence between TGF-β and other pathways that can be specifically targeted for therapy.
Figures
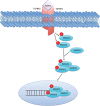
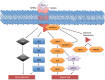
Figure 1.
Canonical transforming growth factor β (TGF-β) signaling. The TGF-β ligand binds its type 2 transmembrane receptor (TGFBR2), recruiting the type 1 receptor (TGFBR1), leading to the phosphorylation of SMAD2 and SMAD3. This phosphorylation event allows for complexing with SMAD4 and nuclear translocation, leading to growth arrest in benign epithelial cells. P = phosphorylation.
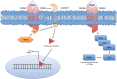
Figure 2.
Mechanisms of transforming growth factor β receptor (TGFBR) repression. Repression of TGFBRs occurs at both the epigenetic and protein levels. It was recently discovered that the ubiquitination of TGFBR1 by tumor necrosis factor receptor associated factor 6 (TRAF6) leads to ADAM metallopeptidase domain 17 (ADAM17) mediated cleavage, liberating the TGFBR1 intracellular domain (ICD). The TGFBR1-ICD is then free to translocate to the nucleus and activate factors including Snail and matrix metalloproteinase 2 (MMP2). Additionally, epigenetic repression of the TGFBRs may be dependent on ERK activation of DNA methyltransferase (DNMT), which hypermethylates the TGFBR promoters, downregulating their expression.
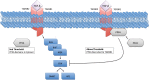
Figure 3.
Transforming growth factor β (TGF-β) control over ERK MAPK. It is hypothesized that in some cells, there is a reciprocal control mechanism involving ERK and the transforming growth factor β receptors (TGFBRs). ERK activation leads to DNA methyltransferase (DNMT) and activator protein (AP1) expression, which is believed to lead to epigenetic repression of the TGFBRs and upregulation of the TGF-β ligand. In some contexts, TGF-β suppresses ERK activation. A key step in this process appears to be recruitment of protein phosphatase 2A (PP2A) to the cell membrane by TGFBR1. In the proposed model, once a sufficient quantity of PP2A is recruited, it serves as a barrier to ERK activation, where DNMT and AP1 expression are not induced, and the canonical SMAD arm of the TGF-βcascade is favored.
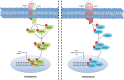
Figure 4.
Transforming growth factor β (TGF-β) in endothelial cell function. The effects of TGF-β on the vascular endothelia are complex and seemingly biphasic. Low TGF-β concentrations appear to activate the activin receptor-like kinase 1 (ALK1)/SMAD1/5 pathway, stimulating endothelial cell (EC) proliferation and migration associated with higher levels of ID1. Yet, at higher levels, TGF-β activates its canonical SMAD2/3 pathway in a transforming growth factor β receptor 1 (TGFBR1; also known as ALK5)–dependent manner to induce antiangiogenic factors plasminiogen activator inhibitor-1 (PAI-1) and fibronectin. P = phosphorylation.
Figure 5.
Aberrant transforming growth factor β (TGF-β) signaling in cancer. TGF-β has several tumor suppressive functions in benign cells. However, these effects are commonly lost in advanced cancers, and TGF-β begins to actively contribute to disease progression. The figure describes a series of partial mechanisms underlying the uncontrolled response to TGF-β in cancer cells and the dysregulation of several associated oncogenic factors leading to enhanced cell proliferation and migration.
References
- Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8(5):341–352 - PubMed
- Akhurst RJ, Derynck R. TGF-beta signaling in cancer—a double-edged sword. Trends Cell Biol. 2001;11(11):S44–S51 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA137000/CA/NCI NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- R01 CA112520/CA/NCI NIH HHS/United States
- U01 CA152738/CA/NCI NIH HHS/United States
- CA126888/CA/NCI NIH HHS/United States
- U01CA152738/CA/NCI NIH HHS/United States
- P50CA090386/CA/NCI NIH HHS/United States
- P50 CA090386/CA/NCI NIH HHS/United States
- R01 CA126888/CA/NCI NIH HHS/United States
- CA112520/CA/NCI NIH HHS/United States
- P30 CA012197/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources