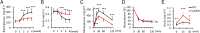Inhibition of p53 preserves Parkin-mediated mitophagy and pancreatic β-cell function in diabetes - PubMed (original) (raw)
Inhibition of p53 preserves Parkin-mediated mitophagy and pancreatic β-cell function in diabetes
Atsushi Hoshino et al. Proc Natl Acad Sci U S A. 2014.
Abstract
Mitochondrial compromise is a fundamental contributor to pancreatic β-cell failure in diabetes. Previous studies have demonstrated a broader role for tumor suppressor p53 that extends to the modulation of mitochondrial homeostasis. However, the role of islet p53 in glucose homeostasis has not yet been evaluated. Here we show that p53 deficiency protects against the development of diabetes in streptozotocin (STZ)-induced type 1 and db/db mouse models of type 2 diabetes. Glucolipotoxicity stimulates NADPH oxidase via receptor for advanced-glycation end products and Toll-like receptor 4. This oxidative stress induces the accumulation of p53 in the cytosolic compartment of pancreatic β-cells in concert with endoplasmic reticulum stress. Cytosolic p53 disturbs the process of mitophagy through an inhibitory interaction with Parkin and induces mitochondrial dysfunction. The occurrence of mitophagy is maintained in STZ-treated p53(-/-) mice that exhibit preserved glucose oxidation capacity and subsequent insulin secretion signaling, leading to better glucose tolerance. These protective effects are not observed when Parkin is deleted. Furthermore, pifithrin-α, a specific inhibitor of p53, ameliorates mitochondrial dysfunction and glucose intolerance in both STZ-treated and db/db mice. Thus, an intervention with cytosolic p53 for a mitophagy deficiency may be a therapeutic strategy for the prevention and treatment of diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
p53 −/− mice were resistant to hyperglycemia in STZ-induced type 1 diabetes. (A) Blood glucose and (B) serum insulin concentrations from mice fed ad libitum were measured at the indicated time points after STZ treatment (n = 16–18). (C) Glucose and (D) insulin tolerance tests were performed 4 wk after STZ treatment (n = 12). (E) Serum insulin values were assessed before and at 15 and 30 min into the glucose tolerance test (n = 12). Data are shown as the means ± SD. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 2.
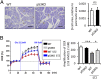
Glucose oxidation capacity was preserved in STZ-treated p53 −/− mice. (A) Histological analysis of insulin immunostaining of islets 4 wk after STZ treatment. (Scale bars, 500 μm.) The β-cell area relative to the pancreatic area was quantified (n = 4). (B) Representative results of the OCR of islets assessed using the Seahorse XF24 extracellular flux analyzer. The amplification rate of OCR at high (22.2 mM) glucose was calculated relative to basal (2.8 mM) glucose stimulation from five replicates per sample (n = 4). Data are shown as the means ± SD. *P < 0.05; ***P < 0.001.
Fig. 3.
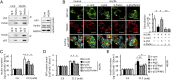
Cytosolic p53 inhibits Parkin-mediated mitophagy in β-cells. (A) The endogenous Parkin–p53 complex in β-cells. Cytosolic lysate of MIN6 β-cells exposed to 30 mM high glucose (HG) and 0.3 mM palmitate (PA) for 1 wk was immunoprecipitated with anti-p53, Parkin, and control IgG antibodies. (B) Representative images of GFP-LC3–overxpressing MIN6 β-cells exposed to HG and PA. Cells were transfected with siRNA targeting p53 and/or Parkin and were treated to underscore mitophagy with 100 nM Baf-A1, a specific inhibitor of H+-ATPases of the vacuolar type, for 6 h before the immunostaining of mitochondria with anti-TOM20 (red) (original magnification, ×1,000). (Scale bars, 10 μm.) (Right) Colocalization between LC3 and TOM20. A minimum of 50 GFP-positive cells were scored in three independent experiments. (C and D) MIN6 β-cells were stimulated with 2.8 or 22.2 mM glucose for 30 min. Insulin secretion (C) and ATP contents (D) were measured by ELISA and a luminometer, respectively, in duplicate (n = 4). (E) Glucose-stimulated insulin secretion was measured by ELISA in the context of mitophagy deficiency induced by the transfected with siRNA targeting Atg5 or PINK1. The experiments were performed in duplicate (n = 4). Data are shown as the means ± SD. *P < 0.05; **P < 0.01.
Fig. 4.
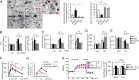
Parkin-mediated mitophagy maintains insulin secretion under diabetic conditions. (A) Construction of the stable MIN6 β-cell lines overexpressing Parkin. Representative immunoblots are shown from three independent experiments. (B) Excitation ratio (590/430 nm) of pMT-mKeima-Red–overexpressing stable cell lines incubated with HG and PA. Baf-A1–treated cells were used as a negative control for the induction of mitophagy. The experiments were performed in eight replicates (n = 3). (C) The sequential state III OCR of isolated mitochondria from stable cell lines was assessed using the Seahorse XF24 analyzer. Representative results are shown, and the OCR of each complex was calculated from five replicates per sample (n = 3). (D) Glucokinase (GK) and hexokinase (HK) activities in stable cell lines. Results are shown from four independent experiments performed in duplicate. (E) ATP contents and (F and G) insulin secretion were measured by a luminometer and ELISA, respectively. The experiments were performed in duplicate (n = 4). Data are shown as the means ± SD. †P < 0.05 versus stable cell lines carrying control vectors under the same condition.
Fig. 5.
Parkin-mediated mitophagy links p53 deficiency and protection against diabetes. (A) Representative electron micrographs of STZ-treated islets. Arrows indicate abnormal mitochondria defined by marked swelling associated with an increased number of disarrayed or disappeared cristae and by the reduced electron density of the matrix (original magnification ×5,000). (Scale bars, 2 μm.) Magnified photograph represents an autophagic vacuole containing mitochondria (red box) (original magnification ×12,000). (Scale bar, 500 nm.) (Right) Abnormal mitochondria and mitochondria incorporated into the autophagic vacuole were quantified from at least 10 β-cells (n = 3). (B and C) Batches of 10 pancreatic islets isolated from STZ-treated WT, p53 −/−, Parkin −/−, and p53 −/− Parkin −/− mice were stimulated with 2.8 or 22.2 mM glucose for 30 min. ATP contents (B) and insulin secretion (C) were measured by a luminometer and ELISA, respectively. The amplification rates of ATP contents and insulin secretion at high glucose were calculated relative to basal glucose stimulation. Results are shown from duplicate experiments per sample (n = 6). (D) Blood glucose levels and (E) serum insulin levels from mice fed ad libitum (n = 11–20). (F) Glucose tolerance tests were performed after 4 wk of PFT-α or the carrier DMSO treatment (n = 12). (G) Serum insulin values were assessed before and at 15 and 30 min into the glucose tolerance test (n = 12). (H) The OCR of islets was assessed using the Seahorse XF24 analyzer. The amplification rate of OCR at high glucose was calculated relative to basal glucose stimulation from five replicates per sample (n = 4). Data are shown as the means ± SD. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 6.
PFT-α preserves β-cell integrity in db/db mice. (A) Six-week-old mice were injected with PFT-α or the carrier DMSO three times per week for 4 wk. Blood glucose levels were measured at the indicated time points (n = 14). (B) Serum insulin levels from mice fed ad libitum were measured after 4 wk of PFT-α or the carrier DMSO treatment (n = 14). (C) Glucose and (D) insulin tolerance tests were performed after 4 wk of treatment (n = 8). (E) Serum insulin values were assessed before and at 15 and 30 min into the glucose tolerance test (n = 8). (F) Representative electron micrographs of islets in db/db mice treated with PFT-α or the carrier DMSO. Arrows indicate abnormal mitochondria (original magnification ×5,000). (Scale bars, 2 μm.) Magnified photograph represents an autophagic vacuole containing mitochondria (red box) (original magnification ×12,000). (Scale bar, 500 nm.) (Lower) Abnormal mitochondria and mitochondria incorporated into the autophagic vacuole were quantified from at least 10 β-cells (n = 3). (G) Batches of five pancreatic db/db mice islets were stimulated with 2.8 or 22.2 mM glucose. The OCR of islets was assessed using the Seahorse XF24 analyzer. The amplification rate of OCR at high glucose was calculated relative to basal glucose stimulation from five replicates per sample (n = 4). (H) ATP contents and (I) insulin secretion were measured by a luminometer and ELISA, respectively. The amplification rates of ATP contents and insulin secretion at high glucose were calculated relative to basal glucose stimulation. Results are shown from duplicate experiments per sample (n = 4). Data are shown as the means ± SD. *P < 0.05; **P < 0.01; ***P < 0.001.
Similar articles
- Metformin Restores Parkin-Mediated Mitophagy, Suppressed by Cytosolic p53.
Song YM, Lee WK, Lee YH, Kang ES, Cha BS, Lee BW. Song YM, et al. Int J Mol Sci. 2016 Jan 16;17(1):122. doi: 10.3390/ijms17010122. Int J Mol Sci. 2016. PMID: 26784190 Free PMC article. - Parkin-mediated mitophagy is negatively regulated by FOXO3A, which inhibits Plk3-mediated mitochondrial ROS generation in STZ diabetic stress-treated pancreatic β cells.
Shim JY, Chung JO, Jung D, Kang PS, Park SY, Kendi AT, Lowe VJ, Lee S. Shim JY, et al. PLoS One. 2023 May 3;18(5):e0281496. doi: 10.1371/journal.pone.0281496. eCollection 2023. PLoS One. 2023. PMID: 37134105 Free PMC article. - Observation of Parkin-Mediated Mitophagy in Pancreatic β-Cells.
Hoshino A, Matoba S. Hoshino A, et al. Methods Mol Biol. 2018;1759:41-46. doi: 10.1007/7651_2017_39. Methods Mol Biol. 2018. PMID: 28466262 - Sirt3 deficiency exacerbates diabetic cardiac dysfunction: Role of Foxo3A-Parkin-mediated mitophagy.
Yu W, Gao B, Li N, Wang J, Qiu C, Zhang G, Liu M, Zhang R, Li C, Ji G, Zhang Y. Yu W, et al. Biochim Biophys Acta Mol Basis Dis. 2017 Aug;1863(8):1973-1983. doi: 10.1016/j.bbadis.2016.10.021. Epub 2016 Oct 26. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 27794418 Review. - Oxidative and endoplasmic reticulum stress in β-cell dysfunction in diabetes.
Hasnain SZ, Prins JB, McGuckin MA. Hasnain SZ, et al. J Mol Endocrinol. 2016 Feb;56(2):R33-54. doi: 10.1530/JME-15-0232. Epub 2015 Nov 17. J Mol Endocrinol. 2016. PMID: 26576641 Review.
Cited by
- Common Principles and Specific Mechanisms of Mitophagy from Yeast to Humans.
Kumar R, Reichert AS. Kumar R, et al. Int J Mol Sci. 2021 Apr 22;22(9):4363. doi: 10.3390/ijms22094363. Int J Mol Sci. 2021. PMID: 33922020 Free PMC article. Review. - A Prospective Study of Leukocyte Telomere Length and Risk of Gestational Diabetes in a Multiracial Cohort.
Lin Y, Zhu Y, Wu J, Hinkle SN, Rawal S, Han J, Weir NL, Tsai MY, Zhang C. Lin Y, et al. Epidemiology. 2019 Nov;30 Suppl 2(Suppl 2):S10-S16. doi: 10.1097/EDE.0000000000001081. Epidemiology. 2019. PMID: 31569148 Free PMC article. - High glucose represses the proliferation of tendon fibroblasts by inhibiting autophagy activation in tendon injury.
Song FC, Yuan JQ, Zhu MD, Li Q, Liu SH, Zhang L, Zhao C. Song FC, et al. Biosci Rep. 2022 Mar 31;42(3):BSR20210640. doi: 10.1042/BSR20210640. Biosci Rep. 2022. PMID: 35293974 Free PMC article. - lncRNA ZEB1-AS1 Was Suppressed by p53 for Renal Fibrosis in Diabetic Nephropathy.
Wang J, Pan J, Li H, Long J, Fang F, Chen J, Zhu X, Xiang X, Zhang D. Wang J, et al. Mol Ther Nucleic Acids. 2018 Sep 7;12:741-750. doi: 10.1016/j.omtn.2018.07.012. Epub 2018 Jul 31. Mol Ther Nucleic Acids. 2018. PMID: 30121551 Free PMC article. - α-Synucleinopathy associated c-Abl activation causes p53-dependent autophagy impairment.
Karim MR, Liao EE, Kim J, Meints J, Martinez HM, Pletnikova O, Troncoso JC, Lee MK. Karim MR, et al. Mol Neurodegener. 2020 Apr 16;15(1):27. doi: 10.1186/s13024-020-00364-w. Mol Neurodegener. 2020. PMID: 32299471 Free PMC article.
References
- Muoio DM, Newgard CB. Mechanisms of disease: Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(3):193–205. - PubMed
- Zhang CY, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105(6):745–755. - PubMed
- Szabadkai G, Duchen MR. Mitochondria mediated cell death in diabetes. Apoptosis. 2009;14(12):1405–1423. - PubMed
- Vousden KH, Ryan KM. p53 and metabolism. Nat Rev Cancer. 2009;9(10):691–700. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous