Mapping the capacities of fixed health facilities to cover people at risk of gambiense human African trypanosomiasis - PubMed (original) (raw)
Mapping the capacities of fixed health facilities to cover people at risk of gambiense human African trypanosomiasis
Pere P Simarro et al. Int J Health Geogr. 2014.
Abstract
Background: The emphasis placed on the activities of mobile teams in the detection of gambiense human African trypanosomiasis (HAT) can at times obscure the major role played by fixed health facilities in HAT control and surveillance. The lack of consistent and detailed data on the coverage of passive case-finding and treatment further constrains our ability to appreciate the full contribution of the health system to the control of HAT.
Methods: A survey was made of all fixed health facilities that are active in the control and surveillance of gambiense HAT. Information on their diagnostic and treatment capabilities was collected, reviewed and harmonized. Health facilities were geo-referenced. Time-cost distance analysis was conducted to estimate physical accessibility and the potential coverage of the population at-risk of gambiense HAT.
Results: Information provided by the National Sleeping Sickness Control Programmes revealed the existence of 632 fixed health facilities that are active in the control and surveillance of gambiense HAT in endemic countries having reported cases or having conducted active screening activities during the period 2000-2012. Different types of diagnosis (clinical, serological, parasitological and disease staging) are available from 622 facilities. Treatment with pentamidine for first-stage disease is provided by 495 health facilities, while for second-stage disease various types of treatment are available in 206 health facilities only. Over 80% of the population at-risk for gambiense HAT lives within 5-hour travel of a fixed health facility offering diagnosis and treatment for the disease.
Conclusions: Fixed health facilities have played a crucial role in the diagnosis, treatment and coverage of at-risk-population for gambiense HAT. As the number of reported cases continues to dwindle, their role will become increasingly important for the prospects of disease elimination. Future updates of the database here presented will regularly provide evidence to inform and monitor a rational deployment of control and surveillance efforts. Support to the development and, if successful, the implementation of new control tools (e.g. new diagnostics and new drugs) is crucial, both for strengthening and expanding the existing network of fixed health facilities by improving access to diagnosis and treatment and for securing a sustainable control and surveillance of gambiense HAT.
Figures
Figure 1
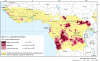
The risk of T. b. gambiense infection in Africa. Adapted from [7]. Risk is based on the reported number of gambiense HAT cases in the period 2000-2009 and on Landscan™ human population layers for the same period [6]. As a backdrop, the predicted distribution of the palpalis group of tsetse flies is provided. The palpalis group (Genus: Glossina, subgenus: Nemorhina) includes all the major vector species of gambiense HAT.
Figure 2
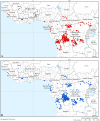
Geographic distribution of fixed health facilities having capacities for gambiense HAT diagnosis (A) and treatment (B). Data were collected by WHO between December 2012 and August 2013 from National Sleeping Sickness Control Programmes in the study countries (i.e. Angola, Benin, Burkina Faso, Cameroon, Central African Republic, Chad, Congo, Côte d’Ivoire, Democratic Republic of the Congo, Equatorial Guinea, Gabon, Ghana, Guinea, Mali, Nigeria, Sierra Leone, South Sudan, Togo and Uganda).
Figure 3
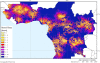
Travel time (or physical accessibility) to health facilities having capabilities for diagnosis of gambiense HAT [hours]. Physical accessibility was calculated through a cost-distance function. Travel cost was measured in time units, and the destinations were represented by health facilities having capabilities for HAT diagnosis (Figure 2A). The time-cost of travel was derived from a ‘friction’ layer for Africa [11].
Similar articles
- Monitoring the elimination of human African trypanosomiasis: Update to 2016.
Franco JR, Cecchi G, Priotto G, Paone M, Diarra A, Grout L, Simarro PP, Zhao W, Argaw D. Franco JR, et al. PLoS Negl Trop Dis. 2018 Dec 6;12(12):e0006890. doi: 10.1371/journal.pntd.0006890. eCollection 2018 Dec. PLoS Negl Trop Dis. 2018. PMID: 30521525 Free PMC article. - Factors influencing passive surveillance for T. b. rhodesiense human african trypanosomiasis in Uganda.
Acup C, Bardosh KL, Picozzi K, Waiswa C, Welburn SC. Acup C, et al. Acta Trop. 2017 Jan;165:230-239. doi: 10.1016/j.actatropica.2016.05.009. Epub 2016 May 19. Acta Trop. 2017. PMID: 27212706 - The Atlas of human African trypanosomiasis: a contribution to global mapping of neglected tropical diseases.
Simarro PP, Cecchi G, Paone M, Franco JR, Diarra A, Ruiz JA, Fèvre EM, Courtin F, Mattioli RC, Jannin JG. Simarro PP, et al. Int J Health Geogr. 2010 Nov 1;9:57. doi: 10.1186/1476-072X-9-57. Int J Health Geogr. 2010. PMID: 21040555 Free PMC article. - Priorities for the elimination of sleeping sickness.
Welburn SC, Maudlin I. Welburn SC, et al. Adv Parasitol. 2012;79:299-337. doi: 10.1016/B978-0-12-398457-9.00004-4. Adv Parasitol. 2012. PMID: 22726645 Review. - Contemporary and emerging strategies for eliminating human African trypanosomiasis due to Trypanosoma brucei gambiense: review.
Steinmann P, Stone CM, Sutherland CS, Tanner M, Tediosi F. Steinmann P, et al. Trop Med Int Health. 2015 Jun;20(6):707-18. doi: 10.1111/tmi.12483. Epub 2015 Mar 12. Trop Med Int Health. 2015. PMID: 25694261 Review.
Cited by
- Proximity of Water Wells to Public Water Testing Facilities in Alberta Using Drive Times.
Munene A, Hall DC. Munene A, et al. Environ Health Insights. 2022 Nov 14;16:11786302221137437. doi: 10.1177/11786302221137437. eCollection 2022. Environ Health Insights. 2022. PMID: 36408333 Free PMC article. - Enhanced passive screening and diagnosis for gambiense human African trypanosomiasis in north-western Uganda - Moving towards elimination.
Wamboga C, Matovu E, Bessell PR, Picado A, Biéler S, Ndung'u JM. Wamboga C, et al. PLoS One. 2017 Oct 12;12(10):e0186429. doi: 10.1371/journal.pone.0186429. eCollection 2017. PLoS One. 2017. PMID: 29023573 Free PMC article. - Cost-effectiveness of using a rapid diagnostic test to screen for human African trypanosomiasis in the Democratic Republic of the Congo.
Bessell PR, Lumbala C, Lutumba P, Baloji S, Biéler S, Ndung'u JM. Bessell PR, et al. PLoS One. 2018 Sep 21;13(9):e0204335. doi: 10.1371/journal.pone.0204335. eCollection 2018. PLoS One. 2018. PMID: 30240406 Free PMC article. - Quantifying epidemiological drivers of gambiense human African Trypanosomiasis across the Democratic Republic of Congo.
Crump RE, Huang CI, Knock ES, Spencer SEF, Brown PE, Mwamba Miaka E, Shampa C, Keeling MJ, Rock KS. Crump RE, et al. PLoS Comput Biol. 2021 Jan 29;17(1):e1008532. doi: 10.1371/journal.pcbi.1008532. eCollection 2021 Jan. PLoS Comput Biol. 2021. PMID: 33513134 Free PMC article. - The impact of passive case detection on the transmission dynamics of gambiense Human African Trypanosomiasis.
Checchi F, Funk S, Chandramohan D, Chappuis F, Haydon DT. Checchi F, et al. PLoS Negl Trop Dis. 2018 Apr 6;12(4):e0006276. doi: 10.1371/journal.pntd.0006276. eCollection 2018 Apr. PLoS Negl Trop Dis. 2018. PMID: 29624584 Free PMC article.
References
- WHO. Human African trypanosomiasis (sleeping sickness): epidemiological update. Wkly Epidemiol Rec. 2006;81(8):71–80. - PubMed
- WHO. Technical Report Series. Geneva: World Health Organization; 2013. Control and surveillance of human African trypanosomiasis. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials