Direct exchange of vitamin B12 is demonstrated by modelling the growth dynamics of algal-bacterial cocultures - PubMed (original) (raw)
Direct exchange of vitamin B12 is demonstrated by modelling the growth dynamics of algal-bacterial cocultures
Matthew A A Grant et al. ISME J. 2014 Jul.
Abstract
The growth dynamics of populations of interacting species in the aquatic environment is of great importance, both for understanding natural ecosystems and in efforts to cultivate these organisms for industrial purposes. Here we consider a simple two-species system wherein the bacterium Mesorhizobium loti supplies vitamin B12 (cobalamin) to the freshwater green alga Lobomonas rostrata, which requires this organic micronutrient for growth. In return, the bacterium receives photosynthate from the alga. Mathematical models are developed that describe minimally the interdependence between the two organisms, and that fit the experimental observations of the consortium. These models enable us to distinguish between different mechanisms of nutrient exchange between the organisms, and provide strong evidence that, rather than undergoing simple lysis and release of nutrients into the medium, M. loti regulates the levels of cobalamin it produces, resulting in a true mutualism with L. rostrata. Over half of all microalgae are dependent on an exogenous source of cobalamin for growth, and this vitamin is synthesised only by bacteria; it is very likely that similar symbiotic interactions underpin algal productivity more generally.
Figures
Figure 1
Vitamin B12-requiring algae are only able to grow in the presence of cobalamin, which can only be synthesised by some bacteria. (a) The structure of cobalamin (vitamin B12). (b) The model laboratory system developed by Kazamia et al. (2012) to study this interaction. L. rostrata, a vitamin B12 auxotroph, grows in the presence of M. loti in autotrophic medium that does not contain cobalamin. Controls indicate that this is because of the exchange of a carbon source for vitamin B12.
Figure 2
The models describe the growth of L. rostrata and M. loti seen in experiments. In all graphs, the symbols represent the experimental data from Kazamia et al. (2012), collected with three biological replicates per time point per treatment, whereas the lines are output of the models. (a) The lines show the trends given by Model 2 at different initial conditions (different starting values of a and b), without add-back of nutrients. The algae (top panel) and bacteria (bottom panel) grow until they reach the carrying capacity. Model 1 gives a similar fit. (b) The equilibrium is broken by adding back either a carbon source in the form of glycerol (magenta lines and symbols) or vitamin B12 (green lines and symbols). The control (i.e. no addition) is shown in blue. The unregulated model (Model 1) recreates the increase in the number of algae when B12 is added to the medium (top panel), and the increase in the number of bacteria when carbon is added to the medium (bottom panel). The regulated model (Model 2) similarly recreates the increase in the number of algae and bacteria after the addition of B12 or carbon, respectively. It is also able to capture the corresponding decrease in algal numbers seen upon the addition of carbon (top panel, magenta line), and bacteria after addition of B12 (bottom panel, green line). Note that the scale of the y axis is logarithmic.
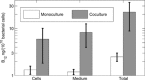
Figure 3
Levels of B12 in monoculture of M. loti versus coculture with L. rostrata. B12 was measured separately in the cell pellet, the medium and the total (i.e. before centrifugation to pellet the cells). In the monoculture, the medium has been supplemented with carbon. In the coculture, the cell fraction includes the L. rostrata cells. The values, normalised to the numbers of bacterial cells, are the average of three biological replicates. Note that the scale is logarithmic.
Similar articles
- Quantitative proteomics of a B12 -dependent alga grown in coculture with bacteria reveals metabolic tradeoffs required for mutualism.
Helliwell KE, Pandhal J, Cooper MB, Longworth J, Kudahl UJ, Russo DA, Tomsett EV, Bunbury F, Salmon DL, Smirnoff N, Wright PC, Smith AG. Helliwell KE, et al. New Phytol. 2018 Jan;217(2):599-612. doi: 10.1111/nph.14832. Epub 2017 Oct 16. New Phytol. 2018. PMID: 29034959 Free PMC article. - Mutualistic interactions between vitamin B12 -dependent algae and heterotrophic bacteria exhibit regulation.
Kazamia E, Czesnick H, Nguyen TT, Croft MT, Sherwood E, Sasso S, Hodson SJ, Warren MJ, Smith AG. Kazamia E, et al. Environ Microbiol. 2012 Jun;14(6):1466-76. doi: 10.1111/j.1462-2920.2012.02733.x. Epub 2012 Mar 29. Environ Microbiol. 2012. PMID: 22463064 - Cryopreservation studies of an artificial co-culture between the cobalamin-requiring green alga Lobomonas rostrata and the bacterium Mesorhizobium loti.
Ridley CJA, Day JG, Smith AG. Ridley CJA, et al. J Appl Phycol. 2018;30(2):995-1003. doi: 10.1007/s10811-017-1270-8. Epub 2017 Sep 27. J Appl Phycol. 2018. PMID: 29755204 Free PMC article. - Eutrophication of freshwater and coastal marine ecosystems: a global problem.
Smith VH. Smith VH. Environ Sci Pollut Res Int. 2003;10(2):126-39. doi: 10.1065/espr2002.12.142. Environ Sci Pollut Res Int. 2003. PMID: 12729046 Review. - Microbial and Genetic Resources for Cobalamin (Vitamin B12) Biosynthesis: From Ecosystems to Industrial Biotechnology.
Balabanova L, Averianova L, Marchenok M, Son O, Tekutyeva L. Balabanova L, et al. Int J Mol Sci. 2021 Apr 26;22(9):4522. doi: 10.3390/ijms22094522. Int J Mol Sci. 2021. PMID: 33926061 Free PMC article. Review.
Cited by
- Tight Adherence (Tad) Pilus Genes Indicate Putative Niche Differentiation in Phytoplankton Bloom Associated Rhodobacterales.
Isaac A, Francis B, Amann RI, Amin SA. Isaac A, et al. Front Microbiol. 2021 Aug 10;12:718297. doi: 10.3389/fmicb.2021.718297. eCollection 2021. Front Microbiol. 2021. PMID: 34447362 Free PMC article. - Extracellular Metabolism Sets the Table for Microbial Cross-Feeding.
Fritts RK, McCully AL, McKinlay JB. Fritts RK, et al. Microbiol Mol Biol Rev. 2021 Jan 13;85(1):e00135-20. doi: 10.1128/MMBR.00135-20. Print 2021 Feb 17. Microbiol Mol Biol Rev. 2021. PMID: 33441489 Free PMC article. Review. - Fundamental shift in vitamin B12 eco-physiology of a model alga demonstrated by experimental evolution.
Helliwell KE, Collins S, Kazamia E, Purton S, Wheeler GL, Smith AG. Helliwell KE, et al. ISME J. 2015 Jun;9(6):1446-55. doi: 10.1038/ismej.2014.230. Epub 2014 Dec 19. ISME J. 2015. PMID: 25526368 Free PMC article. - Quantitative proteomics of a B12 -dependent alga grown in coculture with bacteria reveals metabolic tradeoffs required for mutualism.
Helliwell KE, Pandhal J, Cooper MB, Longworth J, Kudahl UJ, Russo DA, Tomsett EV, Bunbury F, Salmon DL, Smirnoff N, Wright PC, Smith AG. Helliwell KE, et al. New Phytol. 2018 Jan;217(2):599-612. doi: 10.1111/nph.14832. Epub 2017 Oct 16. New Phytol. 2018. PMID: 29034959 Free PMC article. - Extracellular Polymeric Substances Drive Symbiotic Interactions in Bacterial‒Microalgal Consortia.
Perera IA, Abinandan S, Subashchandrabose SR, Venkateswarlu K, Cole N, Naidu R, Megharaj M. Perera IA, et al. Microb Ecol. 2022 Apr;83(3):596-607. doi: 10.1007/s00248-021-01772-1. Epub 2021 Jun 16. Microb Ecol. 2022. PMID: 34132846
References
- Bertrand EM, Saito MA, Rose JM, Riesselman CR, Noble AE, et al. Vitamin B12 and iron colimitation of phytoplankton growth in the Ross Sea. Limnol Oceanogr. 2007;52:1079–1093.
- Coale KH, Johnson KS, Chavez FP, Buesseler KO, Barber RT, Brzezinski MA, et al. Southern Ocean iron enrichment experiment: carbon cycling in high- and low-Si waters. Science. 2004;304:408–414. - PubMed
- Cole JJ, Findlay S, Pace ML. Bacterial production in fresh and saltwater ecosystems: a cross-system overview. Mar Ecol Prog Ser. 1988;43:1–10.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources