Nuclear trafficking in health and disease - PubMed (original) (raw)
Review
Nuclear trafficking in health and disease
Amir Mor et al. Curr Opin Cell Biol. 2014 Jun.
Abstract
In eukaryotic cells, the cytoplasm and the nucleus are separated by a double-membraned nuclear envelope (NE). Thus, transport of molecules between the nucleus and the cytoplasm occurs via gateways termed the nuclear pore complexes (NPCs), which are the largest intracellular channels in nature. While small molecules can passively translocate through the NPC, large molecules are actively imported into the nucleus by interacting with receptors that bind nuclear pore complex proteins (Nups). Regulatory factors then function in assembly and disassembly of transport complexes. Signaling pathways, cell cycle, pathogens, and other physiopathological conditions regulate various constituents of the nuclear transport machinery. Here, we will discuss several findings related to modulation of nuclear transport during physiological and pathological conditions, including tumorigenesis, viral infection, and congenital syndrome. We will also explore chemical biological approaches that are being used as probes to reveal new mechanisms that regulate nucleocytoplasmic trafficking and that are serving as starting points for drug development.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
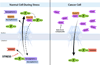
Figure 1. Abnormal Nuclear Export of Proteins in cancer cells
Upon genotoxic stress, various proteins (P) including tumor suppressors, such as p53, accumulate in the nucleus to regulate intranuclear processes. The translocation of these proteins into the nucleus involves recognition of the protein’s nuclear localization sequence (NLS) by a karyopherin or importin, which in some cases bind a second karyopherin. In the nucleus, the karyopherin(s) is dissociated from the cargo through the action of RanGTP. Certain proteins involved in cell proliferation have their NLS masked by inhibitors, which are dissociated upon various stimuli. This effect allows recognition of the NLS by karyopherins and protein import into the nucleus. Some of these proteins also have a nuclear export sequence (NES), which interacts with the export receptor Crm1 (XPO1). This interaction is enhanced by RanGTP, which is followed by subsequent translocation of the export complex to the cytoplasm. In certain types of cancer, Crm1 is overexpressed and promotes nuclear export of proteins, including tumor suppressors, inducing cell proliferation. Anti-Crm1 compounds are being tested for cancer therapeutics.
Figure 2. Disruption of BRCA2-RAD51 nucleocytoplasmic trafficking in cancer cells
During genotoxic stress in normal cells, RAD51 is recruited by BRCA2 to sites of DNA repair. The interaction of BRCA2 with RAD51 masks the RAD51 NES, preventing its export to the cytoplasm. DSS1, which binds BRCA2, masks BRCA2 NES and inhibit Crm1 mediated export. The masking of the two NESs inhibits cytoplasmic redistribution of the BRCA2-RAD51 complex. An abundant mutation in breast cancers (BRCA2D2723H) was found to prevent DSS1 association with BRAC2. As an outcome, BRCA2-NES is unmasked and is exported by Crm1-RanGTP. The remaining nuclear RAD51 are also redistributed to the cytoplasm, preventing its ability to function during DNA damage.
Figure 3. eIF4E-mediated mRNA export and its link to tumorigenesis
eIF4E mediates export of a subset of mRNAs, which contain the 4E-SE RNA element that is recognized by LRPPRC bound to eIF4E. LRPPRC contains an NES that interacts with Crm1-RanGTP, which translocates the mRNP to the cytoplasm. Among the 4E-SE mRNAs, there are important proliferation factors. In many cancer cells, eIF4E and Crm1 levels are elevated resulting in abnormal increase in nuclear export of various mRNAs, including the ones that regulate cell proliferation, which promotes their translation in the cytoplasm. eIF4E up-regulation leads to Nup358 degradation and increased levels of Dbp5, Gle1 and RanBP1. The crosstalk between the bulk mRNA export machinery and the eIF4E mRNA export pathway will be interesting to investigate.
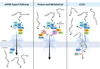
Figure 4. mRNA nuclear export in viral infection, metabolism, and congenital syndrome
Bulk mRNA export is mediated by the TREX complex, which consists of THO, UAP56, and Aly/Ref. The association of Aly with mRNA recruits the mRNA export receptor heterodimer NXF1-NXT1, which mediates export of mRNAs by interacting with Nups at the NPC. Influenza virus NS1 protein or VSV M protein inhibit mRNA export. Low levels of pyrimidine induced by a DHODH inhibitor up-regulates NXF1 and release mRNA export block mediated by these viral proteins. Mutation in the mRNA export factor Gle1 disrupts its function in mRNA export and causes the lethal congenital contracture syndrome-1 (LCCS1).
Similar articles
- Nuclear transport erupts on the slopes of Mount Etna.
Powers MA, Dasso M. Powers MA, et al. Nat Cell Biol. 2004 Feb;6(2):82-6. doi: 10.1038/ncb0204-82. Nat Cell Biol. 2004. PMID: 14755266 - Fifty years of nuclear pores and nucleocytoplasmic transport studies: multiple tools revealing complex rules.
Floch AG, Palancade B, Doye V. Floch AG, et al. Methods Cell Biol. 2014;122:1-40. doi: 10.1016/B978-0-12-417160-2.00001-1. Methods Cell Biol. 2014. PMID: 24857723 Review. - Flexible gates: dynamic topologies and functions for FG nucleoporins in nucleocytoplasmic transport.
Terry LJ, Wente SR. Terry LJ, et al. Eukaryot Cell. 2009 Dec;8(12):1814-27. doi: 10.1128/EC.00225-09. Epub 2009 Oct 2. Eukaryot Cell. 2009. PMID: 19801417 Free PMC article. Review. - Regulation of nuclear transport: central role in development and transformation?
Poon IK, Jans DA. Poon IK, et al. Traffic. 2005 Mar;6(3):173-86. doi: 10.1111/j.1600-0854.2005.00268.x. Traffic. 2005. PMID: 15702986 Review. - Should I stay or should I go? Nucleocytoplasmic trafficking in plant innate immunity.
Wiermer M, Palma K, Zhang Y, Li X. Wiermer M, et al. Cell Microbiol. 2007 Aug;9(8):1880-90. doi: 10.1111/j.1462-5822.2007.00962.x. Epub 2007 May 15. Cell Microbiol. 2007. PMID: 17506817 Review.
Cited by
- Depletion of nuclear import protein karyopherin alpha 7 (KPNA7) induces mitotic defects and deformation of nuclei in cancer cells.
Vuorinen EM, Rajala NK, Ihalainen TO, Kallioniemi A. Vuorinen EM, et al. BMC Cancer. 2018 Mar 27;18(1):325. doi: 10.1186/s12885-018-4261-5. BMC Cancer. 2018. PMID: 29580221 Free PMC article. - Nuclear Export Inhibitor Selinexor Enhances Oncolytic Myxoma Virus Therapy against Cancer.
Rahman MM, van Oosterom F, Enow JA, Hossain M, Gutierrez-Jensen AD, Cashen M, Everts A, Lowe K, Kilbourne J, Daggett-Vondras J, Karr TL, McFadden G. Rahman MM, et al. Cancer Res Commun. 2023 Jun 1;3(6):952-968. doi: 10.1158/2767-9764.CRC-22-0483. eCollection 2023 Jun. Cancer Res Commun. 2023. PMID: 37377603 Free PMC article. - Intrinsically disordered proteins in the nucleus of human cells.
Frege T, Uversky VN. Frege T, et al. Biochem Biophys Rep. 2015 Mar 24;1:33-51. doi: 10.1016/j.bbrep.2015.03.003. eCollection 2015 May. Biochem Biophys Rep. 2015. PMID: 29124132 Free PMC article. - Complex Commingling: Nucleoporins and the Spindle Assembly Checkpoint.
Mossaid I, Fahrenkrog B. Mossaid I, et al. Cells. 2015 Nov 3;4(4):706-25. doi: 10.3390/cells4040706. Cells. 2015. PMID: 26540075 Free PMC article. Review. - The hippo kinase STK38 ensures functionality of XPO1.
Martin AP, Camonis JH. Martin AP, et al. Cell Cycle. 2020 Nov;19(22):2982-2995. doi: 10.1080/15384101.2020.1826619. Epub 2020 Oct 5. Cell Cycle. 2020. PMID: 33017560 Free PMC article. Review.
References
- Köhler A, Hurt E. Exporting RNA from the nucleus to the cytoplasm. Nat. Rev. Mol. Cell Biol. 2007;8:761–773. - PubMed
- Fribourg S, Braun IC, Izaurralde E, Conti E. Structural basis for the recognition of a nucleoporin FG repeat by the NTF2-like domain of the TAP/p15 mRNA nuclear export factor. Mol. Cell. 2001;8:645–656. - PubMed
- Ziegler EC, Ghosh S. Regulating inducible transcription through controlled localization. Sci. STKE. 2005;2005:re6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI089539/AI/NIAID NIH HHS/United States
- R01 AI079110/AI/NIAID NIH HHS/United States
- T32 CA124334/CA/NCI NIH HHS/United States
- R01AI079110/AI/NIAID NIH HHS/United States
- R01AI089539/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources