Rejuvenation of the muscle stem cell population restores strength to injured aged muscles - PubMed (original) (raw)
Rejuvenation of the muscle stem cell population restores strength to injured aged muscles
Benjamin D Cosgrove et al. Nat Med. 2014 Mar.
Abstract
The elderly often suffer from progressive muscle weakness and regenerative failure. We demonstrate that muscle regeneration is impaired with aging owing in part to a cell-autonomous functional decline in skeletal muscle stem cells (MuSCs). Two-thirds of MuSCs from aged mice are intrinsically defective relative to MuSCs from young mice, with reduced capacity to repair myofibers and repopulate the stem cell reservoir in vivo following transplantation. This deficiency is correlated with a higher incidence of cells that express senescence markers and is due to elevated activity of the p38α and p38β mitogen-activated kinase pathway. We show that these limitations cannot be overcome by transplantation into the microenvironment of young recipient muscles. In contrast, subjecting the MuSC population from aged mice to transient inhibition of p38α and p38β in conjunction with culture on soft hydrogel substrates rapidly expands the residual functional MuSC population from aged mice, rejuvenating its potential for regeneration and serial transplantation as well as strengthening of damaged muscles of aged mice. These findings reveal a synergy between biophysical and biochemical cues that provides a paradigm for a localized autologous muscle stem cell therapy for the elderly.
Figures
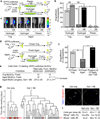
Figure 1. Aged MuSCs have diminished regenerative and self-renewal functions revealing an inherent stem cell defect
(a) MuSC intramuscular transplantation scheme. (b–e) Bioluminescence imaging (BLI) and immunohistology of 10-cell primary transplants from young (Yng) or aged GFP/Luciferase MuSC donors. (b) BLI signals from n = 34 recipients from three experiments. p, photons. Engraftment threshold (dashed line) corresponding to histological detection of ≥1 donor-derived (GFP+) myofibers in (b) and (i). Representative BLI (c) and immunohistological (d) images. Scale bar, 500 µm. (e) GFP+ myofibers per recipient tibialis anterior muscle (n = 6–7 recipients from three experiments) in engrafted samples. (f) Limiting dilution analysis relating primary MuSCs transplanted with percent engraftment from n = 5–34 transplants per condition. (g, h) Flow cytometric analysis of GFP+ fraction of all CD34+α7-integrin+ MuSCs in primary recipients (n = 3, mean ± s.e.m.) transplanted with 100 primary (1°) young or aged MuSCs. (i, j) BLI analysis of secondary recipients transplanted with re-isolated GFP+CD34+α7-integrin+ cells from 700 primary young or aged MuSC recipients. (i) BLI scatter plot (mean overlaid, n = 3). (j) Representative BLI images. P < 0.05 (*) or P < 0.01 (**) by Fisher’s exact test in (b), Mann-Whitney test in (e), unpaired t test in (h), and paired t test in (i).
Figure 2. Aged MuSCs are characterized by a senescence phenotype and elevated p38α/β MAPK signaling
(a) Single-cell RT-PCR analysis of muscle stem cell (Pax7 and Myf5) and myogenic commitment gene (Pax3 and MyoD) expression in purified young (Yng) or aged MuSCs (n = 47 and 33 cells, respectively, from two experiments). (b, c) Clonal division assay. (b) Representative brightfield images of non-dividing and dividing clones. Scale bar, 100 µm. (c) Proportion of young or aged MuSCs that do not undergo clonal division in hydrogel microwell cultures (mean ± s.e.m. of n = 4 experiments). (d, e) Immunostaining analysis of p16Ink4a and p21Cip1 senescence marker expression in purified young and aged MuSCs, with Topro3 nuclear counter-staining. (d) Six representative cells per condition. Positive cells marked with arrowheads. Scale bar, 10 µm. (e) Fraction expressing both p16Ink4a and p21Cip1 (mean ± s.e.m. of n = 3 experiments). (f, g) Flow cytometric analysis of phospho-p38α/β MAPK in purified young or aged MuSCs. (f) Representative histograms. (g) Mean ± s.e.m. of n = 3 experiments. Not significant (NS), P < 0.05 (*) or P < 0.01 (**) by Fisher’s exact test in (a) and Student’s t test in (c, e, g).
Figure 3. p38α/β MAPK inhibition induces proliferation and augments stem-cell gene expression in aged MuSCs in soft hydrogel cultures
(a–i) Analyses of young (Yng) or aged MuSCs after culture on plastic or soft hydrogels with the p38α/β inhibitor SB202190 (SB; 10 µM unless noted) or DMSO control (–), or with an siRNA pool targeting p38α and p38β or a scrambled control (Scr). Arrowheads denote 10 µM in (b, c, h). (a, b) HSP27 and cJun phosphoprotein flow cytometry analyses. (a) Representative phospho-HSP27 histograms. (b) Fraction of phospho-HSP27 or phospho-cJun positive cells (mean ± s.e.m. of n = 3 culture replicates from one experiment. (c) Bulk proliferation analyses (mean ± s.e.m. from n = 3–6 culture replicates from one experiment). (d) Clonal division analysis (mean ± s.e.m. of n = 4 experiments). (e, f) p16Ink4a immunostaining analysis. (e) Representative images. Arrowheads, positive cells. Scale bar, 100 µm. (f) Fraction of cells expressing p16Ink4a (mean ± s.e.m. of n = 4 culture replicates). (g, h) RT-qPCR analysis of Pax7 and Myogenin expression. (g) Mean ± s.e.m of n = 6 culture replicates from two experiments. (h) Mean ± s.e.m of n = 3 culture replicates from one experiment. (i) Flow cytometric analysis of Pax7 and phospho-HSP27. Left, representative scatter plots. Right, p-HSP27+ incidence in Pax7+/− fractions (mean ± s.e.m of n = 3 culture replicates from one experiment). Not significant (NS), P < 0.05 (*) or P < 0.01 (**) for conditions different than control by t test in (b–d, f–i). (j) Summary of SB effects on aged MuSCs in hydrogel cultures.
Figure 4. p38α/β inhibition and soft hydrogel substrate synergistically increase the total yield of functional aged stem cells
(a–c) Engraftment analysis of 100-cell transplants of young (Yng) or aged GFP/Luciferase MuSC population after culture on plastic or hydrogel with SB202190 (SB; 10 µM) or DMSO control (–) into NOD/SCID hindlimbs by bioluminescence imaging (BLI). (a) Transplantation scheme. (b) Percentage of transplants above engraftment threshold (n = 41, 44, 63, 32, 10, and 10 per condition from three experiments). (c) Representative BLI images. p, photons. (d–f) Engraftment analysis of transplants of the total cell yield of 10 aged MuSCs after SB/hydrogel culture or 10 young or 10 aged freshly isolated MuSCs. (d) Transplant scheme. (e) Estimated number of functional MuSCs per condition based GFP/Luciferase MuSC proliferation in culture (n = 3, mean ± s.e.m) and functional MuSC frequencies calculated in Supplementary Fig. 7b. (f) Percentage of transplants above engraftment threshold (n = 24 from one experiment). (g) Representative fate trees for single aged MuSCs in hydrogel culture without or with SB treatment (n = 64 and 75 clones from two experiments). ‘X’ indicates cell death. (h) Corresponding Myogenin (Myog) immunostaining images. Arrowheads, positive nuclei. Scale bar, 100 µm. Microwell boundary, dashed line. (i) Total and Myogenin− cell numbers and percentage of Myogenin+ cells per dividing clone (median ± s.e.m). Not significant (NS), P = 0.08 (#) or P < 0.05 (*) by Fisher's exact test in (b) and (f) or Mann-Whitney test in (i).
Figure 5. _Ex vivo_-expanded aged MuSC progeny reconstitute the stem cell reserve in vivo and serially transplant
(a, b, d–f) Analyses of stem cell repopulation in primary recipients and transplant engraftment in secondary recipients comparing 200 freshly isolated aged GFP/Luciferase MuSCs and the progeny of 200 aged MuSCs after culture on hydrogel ± SB202190 (SB; 10 µM). (a) Serial transplant scheme. (b) Frequency of donor-derived (GFP+) cells in the CD34+α7-integrin+ MuSC population in primary recipient muscles (n = 8–10 per condition). Box-and-whisker plot with median line. (c) Immunohistochemical (IHC) analysis of representative transverse section from notexin-injured muscles transplanted with the progeny of 100 aged Myf5 nLacZ/+ MuSCs after culture on hydrogel with 10 µM SB. Arrowhead indicates Myf5–β-gal+ cell in satellite position. Scale bar, 50 µm. (d–f) Engraftment analysis of secondary transplant recipients (n = 4). (d) BLI signals for up to 2 months after transplant (mean ± s.e.m.). Dashed line represents engraftment detection threshold. p, photons. (e) Representative BLI images at 4 weeks. Engraftment ratios are noted. (f) GFP immunohistochemistry (IHC) of transverse section from representative secondary recipient of SB/hydrogel aged MuSC progeny primary transplant. Scale bar, 75 µm. P < 0.05 (*) or P < 0.001 (***) by unpaired t test in (b) or Fisher’s exact test in (e).
Figure 6. Restoration of muscle strength in injured aged mice by transplantation of _ex vivo_-expanded aged MuSC progeny
(a–e) Engraftment analysis of 200 fresh C57BL/6 aged MuSC or their progeny after culture on hydrogel ± SB202190 (SB; 10 µM) transplanted into syngeneic injured aged recipient muscles. (a) Transplant scheme. (b) Estimated number of functional MuSCs per condition based C57BL/6 MuSC proliferation in culture (n = 3, mean ± s.e.m) and functional MuSC frequencies calculated in Supplementary Fig. 7b. (c) Transplant engraftment frequency from bioluminescence imaging (n = 10 recipients from two experiments). (d–e) Luciferase immunohistochemistry in transverse muscle sections. (d) Representative images. Scale bar, 500 µm. (e) Box-and-whisker plot with median line of percent Luciferase+ myofibers (n = 7 per condition). ND, none detected. (f) In vivo muscle contractile force assay scheme. (g) Representative raw muscle twitch forces in young (Y) and aged (A) mice, without (−) or with (+) notexin injury and subsequent aged MuSC transplantation. (h) Specific muscle twitch forces (mean ± s.e.m. of n = 5 muscles per condition). P < 0.05 (*) or P < 0.001 (***) by Fisher’s exact test in (c), Mann-Whitney test in (e), or t test in (h).
Comment in
- Rejuvenating aged muscle stem cells.
Bentzinger CF, Rudnicki MA. Bentzinger CF, et al. Nat Med. 2014 Mar;20(3):234-5. doi: 10.1038/nm.3499. Nat Med. 2014. PMID: 24603790 No abstract available. - Highlights from the latest articles in regenerative medicine.
Sachlos E, Kerstetter-Fogle A. Sachlos E, et al. Regen Med. 2014;9(4):423-5. doi: 10.2217/rme.14.44. Regen Med. 2014. PMID: 25159059 No abstract available.
Similar articles
- Rejuvenating aged muscle stem cells.
Bentzinger CF, Rudnicki MA. Bentzinger CF, et al. Nat Med. 2014 Mar;20(3):234-5. doi: 10.1038/nm.3499. Nat Med. 2014. PMID: 24603790 No abstract available. - p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice.
Bernet JD, Doles JD, Hall JK, Kelly Tanaka K, Carter TA, Olwin BB. Bernet JD, et al. Nat Med. 2014 Mar;20(3):265-71. doi: 10.1038/nm.3465. Epub 2014 Feb 16. Nat Med. 2014. PMID: 24531379 Free PMC article. - Co-delivery of Wnt7a and muscle stem cells using synthetic bioadhesive hydrogel enhances murine muscle regeneration and cell migration during engraftment.
Han WM, Mohiuddin M, Anderson SE, García AJ, Jang YC. Han WM, et al. Acta Biomater. 2019 Aug;94:243-252. doi: 10.1016/j.actbio.2019.06.025. Epub 2019 Jun 19. Acta Biomater. 2019. PMID: 31228633 Free PMC article. - Stem Cell Aging in Skeletal Muscle Regeneration and Disease.
Yamakawa H, Kusumoto D, Hashimoto H, Yuasa S. Yamakawa H, et al. Int J Mol Sci. 2020 Mar 6;21(5):1830. doi: 10.3390/ijms21051830. Int J Mol Sci. 2020. PMID: 32155842 Free PMC article. Review. - The central role of muscle stem cells in regenerative failure with aging.
Blau HM, Cosgrove BD, Ho AT. Blau HM, et al. Nat Med. 2015 Aug;21(8):854-62. doi: 10.1038/nm.3918. Nat Med. 2015. PMID: 26248268 Free PMC article. Review.
Cited by
- Human muscle stem cells are refractory to aging.
Novak JS, Mázala DAG, Nearing M, Hindupur R, Uapinyoying P, Habib NF, Dickson T, Ioffe OB, Harris BT, Fidelia-Lambert MN, Rossi CT, Hill DA, Wagner KR, Hoffman EP, Partridge TA. Novak JS, et al. Aging Cell. 2021 Jul;20(7):e13411. doi: 10.1111/acel.13411. Epub 2021 Jun 5. Aging Cell. 2021. PMID: 34089289 Free PMC article. - Interactions between muscle stem cells, mesenchymal-derived cells and immune cells in muscle homeostasis, regeneration and disease.
Farup J, Madaro L, Puri PL, Mikkelsen UR. Farup J, et al. Cell Death Dis. 2015 Jul 23;6(7):e1830. doi: 10.1038/cddis.2015.198. Cell Death Dis. 2015. PMID: 26203859 Free PMC article. Review. - Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia.
Fukawa T, Yan-Jiang BC, Min-Wen JC, Jun-Hao ET, Huang D, Qian CN, Ong P, Li Z, Chen S, Mak SY, Lim WJ, Kanayama HO, Mohan RE, Wang RR, Lai JH, Chua C, Ong HS, Tan KK, Ho YS, Tan IB, Teh BT, Shyh-Chang N. Fukawa T, et al. Nat Med. 2016 Jun;22(6):666-71. doi: 10.1038/nm.4093. Epub 2016 May 2. Nat Med. 2016. PMID: 27135739 - The effect of age on stem cell function and utility for therapy.
Narbonne P. Narbonne P. Cell Med. 2018 Jun 8;10:2155179018773756. doi: 10.1177/2155179018773756. eCollection 2018. Cell Med. 2018. PMID: 32634187 Free PMC article. Review. - Regulation of Muscle Satellite Cell Function in Tissue Homeostasis and Aging.
Sacco A, Puri PL. Sacco A, et al. Cell Stem Cell. 2015 Jun 4;16(6):585-7. doi: 10.1016/j.stem.2015.05.007. Cell Stem Cell. 2015. PMID: 26046757 Free PMC article.
References
- Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008;9:213–228. - PubMed
- Grounds MD. Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann N Y Acad Sci. 1998;854:78–91. - PubMed
- Benny Klimek ME, et al. Acute inhibition of myostatin-family proteins preserves skeletal muscle in mouse models of cancer cachexia. Biochem Biophys Res Commun. 2010;391:1548–1554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99 AR061465/AR/NIAMS NIH HHS/United States
- U01 HL100397/HL/NHLBI NIH HHS/United States
- U01 HL099997/HL/NHLBI NIH HHS/United States
- T32 CA009151/CA/NCI NIH HHS/United States
- R21 AG044815/AG/NIA NIH HHS/United States
- R25CA118681/CA/NCI NIH HHS/United States
- R01 AG020961/AG/NIA NIH HHS/United States
- R01 AG009521/AG/NIA NIH HHS/United States
- U01HL100397/HL/NHLBI NIH HHS/United States
- R01 HL096113/HL/NHLBI NIH HHS/United States
- R01AG020961/AG/NIA NIH HHS/United States
- K99AR061465/AR/NIAMS NIH HHS/United States
- T32CA009151/CA/NCI NIH HHS/United States
- R01HL096113/HL/NHLBI NIH HHS/United States
- K99 AG042491/AG/NIA NIH HHS/United States
- U01HL099997/HL/NHLBI NIH HHS/United States
- R25 CA118681/CA/NCI NIH HHS/United States
- R01AG009521/AG/NIA NIH HHS/United States
- K99AG042491/AG/NIA NIH HHS/United States
- R01 AR063963/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous