DNA-guided DNA interference by a prokaryotic Argonaute - PubMed (original) (raw)
. 2014 Mar 13;507(7491):258-261.
doi: 10.1038/nature12971. Epub 2014 Feb 16.
Affiliations
- PMID: 24531762
- PMCID: PMC4697943
- DOI: 10.1038/nature12971
DNA-guided DNA interference by a prokaryotic Argonaute
Daan C Swarts et al. Nature. 2014.
Abstract
RNA interference is widely distributed in eukaryotes and has a variety of functions, including antiviral defence and gene regulation. All RNA interference pathways use small single-stranded RNA (ssRNA) molecules that guide proteins of the Argonaute (Ago) family to complementary ssRNA targets: RNA-guided RNA interference. The role of prokaryotic Ago variants has remained elusive, although bioinformatics analysis has suggested their involvement in host defence. Here we demonstrate that Ago of the bacterium Thermus thermophilus (TtAgo) acts as a barrier for the uptake and propagation of foreign DNA. In vivo, TtAgo is loaded with 5'-phosphorylated DNA guides, 13-25 nucleotides in length, that are mostly plasmid derived and have a strong bias for a 5'-end deoxycytidine. These small interfering DNAs guide TtAgo to cleave complementary DNA strands. Hence, despite structural homology to its eukaryotic counterparts, TtAgo functions in host defence by DNA-guided DNA interference.
Figures
Extended Data Figure 1. Analyses of TtAgo in T. thermophilus and E.coli
a, TtAgo decreases plasmid transformation efficiency of T. thermophilus. Transformation efficiency of different ago mutant strains relative to the transformation efficiency of wild-type strain HB27. HB27EC is an HB27 mutant selected for high competence, and HB27Δ_ago_ is an ago gene knockout strain (Fig. 1a). Strains were transformed with plasmid pMK184 (Extended Data Table 5). Transformations were performed in biological duplicates for each strain. Error bars indicate standard deviations. b, Effect on TtAgo expression on plasmid content in E. coli KRX. TtAgo and TtAgoDM were expressed in E. coli KRX from plasmid pWUR702 and pWUR703. Plasmids were purified from biological triplicate cultures in which expression was induced (+) or not induced (−). Compared with TtAgoDM expression, TtAgo expression in E. coli KRX does not lead to reduced plasmid content. Changes in plasmid yield between induced and not induced cultures probably originate from protein expression energy costs. Error bars indicate standard deviations. c, 10–150-nucleotide (nt) RNA with 5′-OH group co-purifies with TtAgo. 15% denaturing polyacrylamide gels with nucleic acids co-purified with TtAgo and TtAgoDM. Nucleic acids are phosphorylated in a T4 PNK forward reaction (5′-OH groups, and to a lesser extend 5′-P groups, are labelled) using [γ-32P] ATP, and resolved on 15% denaturing polyacrylamide gels. Nucleic acids were not treated (lane 1, 5), RNaseA treated (lanes 2, 6), DNaseI treated (lane 3, 7) or Nuclease P1 treated (lane 4, 8).
Extended Data Figure 2. Change in transcription of T. thermophilus genes after ago gene knockout
a, b, RNA-seq analysis was performed on biological triplicates for each strain. Change in gene expression of genes encoded on the chromosome (a) or on the megaplasmid (b) is shown as the log2 of the fold difference in expression of the average of normalized mapped reads on that gene in HB27Δ_ago_ compared with the average of normalized mapped reads on that gene in HB27. Peaks corresponding to genes involved in host defence are coloured red, whereas peaks corresponding to genes involved in competence are coloured blue. c, Genes or operons containing genes with a log2 expression change greater than 2 or −2.
Extended Data Figure 3. TtAgo cleaves ssDNA using ssDNA guides
a, 21-nucleotide (nt) DNA and RNA guides are complementary to the 45-nucleotide DNA targets. Predicted cleavage positions are indicated with a black triangle. b, 20% denaturing polyacrylamide gel loaded with samples in which TtAgo and TtAgoDM were provided with an RNA or an DNA guide to cleave a 45-nucleotide ssDNA target. c, 21-nucleotide RV and FW DNA guides are complementary to the 98-nucleotide ssDNA targets. Predicted cleavage positions are indicated with a black triangle. d, 98-nucleotide ssDNA targets are incubated with TtAgo and TtAgoDM, provided with complementary and non-complementary DNA guides, and resolved on 15% denaturing polyacrylamide gels.
Extended Data Figure 4. Effect of variation of the 5′-end deoxynucleoside of the siDNA and effect of the temperature on TtAgo cleavage efficiency
a–d, Cleavage of 98-nucleotide ssDNA target (Extended Data Fig. 3c) by TtAgo loaded with complementary siDNAs containing a different 5′ deoxynucleoside, as shown in red. The concentrations of each siDNA were varied (indicated on top of the gels). Products of the reaction were resolved on 15% denaturing polyacrylamide gels. e, TtAgo expression plasmid pWUR702 (no guides added) incubated with TtAgo and TtAgoDM at different temperatures. f, pWUR708 plasmid (FW and RV guides added; Fig. 4b) incubated with TtAgo and TtAgoDM at different temperatures, resolved on 0.8% agarose gels. LIN, linear; M1, 1 kb Generuler marker (Fermentas); OC, open circular; SC, supercoiled. g, 98-nucleotide RV target cleavage (FW guide added) incubated with TtAgo and TtAgoDM at different temperatures, resolved on a 15% denaturing acrylamide gel. M2, O'RangeRuler 5 bp DNA Ladder (Thermo Scientific).
Extended Data Figure 5. Activity analyses of TtAgo
a, b, AT-rich (17% GC) insert of pWUR704 (a) and GC-rich insert (59% GC) of pWUR705 (b). The target sequence is boxed. Restriction sites HindIII and BsmI are indicated in grey. Sequences are displayed in the 3′–5′ direction to allow comparison with Fig. 4b, which shows guide base pairing to this sequence. c, d, SpeI-linearized plasmid pWUR704 (c) and pWUR705 (d) incubated with TtAgo–siDNA and TtAgoDM–siDNA complexes targeting both strands of the plasmid, and resolved on 0.8% agarose gels. LIN, linear; M1, 1 kb Generuler marker (Fermentas); M2, open circular and linearized pWUR704 (c), or open circular and linearized pWUR705 (d); OC, open circular. FW guide: BG3466. RV guide: BG4017. High salt concentration (250 mM NaCl) in the reaction buffer cause the TtAgo-treated samples to run higher in the gel than M1 and M2. e, Two-step plasmid cleavage. Target pWUR704 was first nicked by a TtAgo–siDNA complex targeting the first strand (FW guide, lane 1), after which a TtAgo–siDNA complex targeting the other strand was added (RV guide, lane 2). FW guide is still present, and its presence is therefore indicated as (+). LIN, linear; M1, 1 kb Generuler marker (Fermentas); OC, open circular; SC, supercoiled. f, g, Nb.BsmI-nicked plasmid pWUR704 (f) and pWUR705 (g) incubated with TtAgo–siDNA and TtAgoDM–siDNA complexes targeting the un-nicked strands of the plasmid (33 bp away from the nicking site), and resolved on 0.8% agarose gels. LIN, linear; M1, 1 kb Generuler marker (Fermentas); M2, open circular and linearized pWUR704 (a), or open circular and linearized pWUR705 (b); OC, open circular. High salt concentrations (250 mM NaCl) in the reaction buffer cause the TtAgo-treated samples to run higher in the gel than M1 and M2. h, TtAgo dsDNA cleavage site analysis. (i) Plasmid pWUR704 with TtAgo–siDNA target sequences. Predicted cleavage sites are indicated with black triangles. (ii) pWUR704 was linearized using TtAgo–siDNA complexes targeting the plasmid on both strands. (iii) The linearized plasmid was cleaved using either NheI (as shown) or XbaI (not shown). (iv) Restriction site overhangs and possible overhangs resulting from TtAgo–siDNA cleavage were filled using Klenow fragment polymerase (Fermentas). (v) Blunt-end DNA was ligated using T4 DNA ligase (Fermentas), after which the plasmid could be transformed and later sequenced to determine the cleavage site. Sequences revealed that TtAgo–siDNA complexes indeed cleaved the target at the predicted locations (as shown in a), and are shown in more detail in Fig. 4b and Extended Data Fig. 5a, b. Note that in this picture target sequences are displayed in reversed order compared with Fig. 4b and Extended Data Fig. 5a, b. j, TtAgo prefers Mn2+ over Mg2+ as a divalent cation for cleavage. (i) 21-nucleotide DNA guide and 98-nucleotide ssDNA target used. The predicted cleavage site is indicated with a black triangle. (ii) 98-nucleotide ssDNA target cleavage reaction with TtAgo loaded with a 21-nucleotide siDNA in the presence of increasing concentrations of Mg2+, as indicated on top of the gel. (iii) 98-nucleotide ssDNA target cleavage reaction with TtAgo loaded with a 21-nucleotide siDNA in the presence of increasing concentrations of Mn2+, as indicated. Samples were resolved on 15% denaturing polyacrylamide gels.
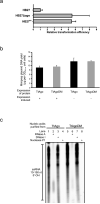
Figure 1. TtAgo interferes with plasmid DNA
a, Overview of ago gene loci of T. thermophilus strains: HB27 (wild type), HB27EC (spontaneous derivative with enhanced competence), HB27Δ_ago_ (knockout), and HB27Δ_ago::sago_ (HB27Δ_ago_ complemented with a strep(II)-tag-ago gene fusion insert). KanR, kanamycin resistance marker. b, Transformation efficiency of T. thermophilus strains on transformation with the plasmid pMHPnqosGFP (Extended Data Table 5). Error bars indicate standard deviations of biological duplicates. c, Yield of pMHPnqosGFP plasmid mini preparation (miniprep) of HB27 and HB27Δ_ago_. Error bars indicate standard deviations of biological triplicates. d, Plasmid content of total DNA purified from HB27Δ_ago_ relative to that from HB27, as quantified by Genetools (Syngene) after resolving the DNA on a 0.8% agarose gel. Error bars indicate standard deviations of biological triplicates.
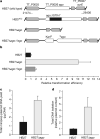
Figure 2. TtAgo guides are 5′-phosphorylated DNA molecules
a, Schematic representation of TtAgo and TtAgoDM proteins used for all experiments (N, PAZ, MID, and PIWI are structural domains, L1 and L2 are linkers). The amino-terminal Strep(II)-tag is indicated as a black square. b, Co-purified nucleic acids from TtAgo and TtAgoDM are labelled with [γ-32P]ATP after phosphate exchange by PNK from bacteriophage T4, and treated with enzymes as indicated. M, custom ssDNA marker; nt, nucleotides. c, Length distribution of unique ssDNA sequences co-purified with TtAgo. d, Nucleotide composition of unique ssDNA sequences co-purified with TtAgo. e, Unique reads of TtAgo co-purified ssDNA molecules mapped on the TtAgo expression vector pWUR702.
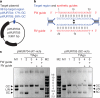
Figure 3. TtAgo cleaves plasmids complementary to its guides
a, b, Untreated target plasmid (lane 1, 5), plasmid incubated at 75 °C in the absence of proteins (lane 2, 6), or in the presence of TtAgoDM (lane 3, 7) or TtAgo (lane 4, 8) purified from E. coli, resolved on 0.8% agarose gels. LIN, linear; M1, 1 kb Generuler marker (Fermentas); M2, linearized and untreated target plasmid; OC, open circular; SC, supercoiled plasmid. a, TtAgo expression vector pWUR702. b, Target plasmid pWUR708, which shares no sequence identity with expression vector pWUR702 or pRARE. Additionally, synthetic (Syn.) ssDNA guides were added to the reactions with pWUR708 (lane 5–8).
Figure 4. TtAgo cleaves plasmids by nicking two strands
a, Plasmids pWUR704 and pWUR705 contain a 98 bp target region with a GC content of 17% or 59%, respectively, as indicated in blue (for details, see Extended Data Fig. 5a, b). b, Part of the pWUR704 and pWUR705 target site (indicated in blue) and complementary ssDNA guides used in this experiment (indicated in red). Black triangles indicate predicted cleavage sites. c, 0.8% agarose gels loaded with pWUR704 and pWUR705 plasmids that were incubated without proteins (lane 1), or with TtAgo (lane 2), TtAgo–forward (FW) guide complex (lane 3), TtAgo–reverse (RV) guide complex (lane 4), or TtAgo–FW and TtAgo–RV guide complexes. LIN, linear; M1, open circular and linear pWUR704 or pWUR705; M2, 1 kb Generuler marker (Fermentas); OC, open circular; SC, supercoiled plasmid.
Comment in
- Biochemistry. A bacterial seek-and-destroy system for foreign DNA.
Vogel J. Vogel J. Science. 2014 May 30;344(6187):972-3. doi: 10.1126/science.1252962. Science. 2014. PMID: 24876480 No abstract available. - Prokaryotic Argonautes - variations on the RNA interference theme.
van der Oost J, Swarts DC, Jore MM. van der Oost J, et al. Microb Cell. 2014 Apr 15;1(5):158-159. doi: 10.15698/mic2014.05.144. Microb Cell. 2014. PMID: 28357239 Free PMC article.
Similar articles
- Effects of Argonaute on Gene Expression in Thermus thermophilus.
Swarts DC, Koehorst JJ, Westra ER, Schaap PJ, van der Oost J. Swarts DC, et al. PLoS One. 2015 Apr 22;10(4):e0124880. doi: 10.1371/journal.pone.0124880. eCollection 2015. PLoS One. 2015. PMID: 25902012 Free PMC article. - Biochemistry. A bacterial seek-and-destroy system for foreign DNA.
Vogel J. Vogel J. Science. 2014 May 30;344(6187):972-3. doi: 10.1126/science.1252962. Science. 2014. PMID: 24876480 No abstract available. - Autonomous Generation and Loading of DNA Guides by Bacterial Argonaute.
Swarts DC, Szczepaniak M, Sheng G, Chandradoss SD, Zhu Y, Timmers EM, Zhang Y, Zhao H, Lou J, Wang Y, Joo C, van der Oost J. Swarts DC, et al. Mol Cell. 2017 Mar 16;65(6):985-998.e6. doi: 10.1016/j.molcel.2017.01.033. Epub 2017 Mar 2. Mol Cell. 2017. PMID: 28262506 Free PMC article. - Understanding the core of RNA interference: The dynamic aspects of Argonaute-mediated processes.
Zhu L, Jiang H, Sheong FK, Cui X, Wang Y, Gao X, Huang X. Zhu L, et al. Prog Biophys Mol Biol. 2017 Sep;128:39-46. doi: 10.1016/j.pbiomolbio.2016.09.008. Epub 2016 Sep 30. Prog Biophys Mol Biol. 2017. PMID: 27697475 Review. - Prokaryotic Argonaute proteins: novel genome-editing tools?
Hegge JW, Swarts DC, van der Oost J. Hegge JW, et al. Nat Rev Microbiol. 2018 Jan;16(1):5-11. doi: 10.1038/nrmicro.2017.73. Epub 2017 Jul 24. Nat Rev Microbiol. 2018. PMID: 28736447 Review.
Cited by
- A thermostable DNA primase-polymerase from a mobile genetic element involved in defence against environmental DNA.
García-Quintans N, Baquedano I, Blesa A, Verdú C, Berenguer J, Mencía M. García-Quintans N, et al. Environ Microbiol. 2020 Nov;22(11):4647-4657. doi: 10.1111/1462-2920.15207. Epub 2020 Sep 3. Environ Microbiol. 2020. PMID: 32830367 Free PMC article. - Programmable endonuclease combined with isothermal polymerase amplification to selectively enrich for rare mutant allele fractions.
Chen J, Qiud T, Mauk MG, Su Z, Fan Y, Yuan DJ, Zhou Q, Qiao Y, Bau HH, Ying J, Song J. Chen J, et al. Chin Chem Lett. 2022 Aug;33(8):4126-4132. doi: 10.1016/j.cclet.2021.11.065. Epub 2021 Nov 26. Chin Chem Lett. 2022. PMID: 36091579 Free PMC article. - Argonaute proteins: structures and their endonuclease activity.
Jin S, Zhan J, Zhou Y. Jin S, et al. Mol Biol Rep. 2021 May;48(5):4837-4849. doi: 10.1007/s11033-021-06476-w. Epub 2021 Jun 11. Mol Biol Rep. 2021. PMID: 34117606 Review. - Target DNA-dependent activation mechanism of the prokaryotic immune system SPARTA.
Finocchio G, Koopal B, Potocnik A, Heijstek C, Westphal AH, Jinek M, Swarts DC. Finocchio G, et al. Nucleic Acids Res. 2024 Feb 28;52(4):2012-2029. doi: 10.1093/nar/gkad1248. Nucleic Acids Res. 2024. PMID: 38224450 Free PMC article. - Distribution of specific prokaryotic immune systems correlates with host optimal growth temperature.
Olijslager LH, Weijers D, Swarts DC. Olijslager LH, et al. NAR Genom Bioinform. 2024 Aug 20;6(3):lqae105. doi: 10.1093/nargab/lqae105. eCollection 2024 Sep. NAR Genom Bioinform. 2024. PMID: 39165676 Free PMC article.
References
- Ketting RF. microRNA biogenesis and function: an overview. Adv. Exp. Med. Biol. 2011;700:1–14. - PubMed
- Averhoff B. Shuffling genes around in hot environments: the unique DNA transporter of Thermus thermophilus. FEMS Microbiol. Rev. 2009;33:611–626. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials