A non-canonical initiation site is required for efficient translation of the dendritically localized Shank1 mRNA - PubMed (original) (raw)
A non-canonical initiation site is required for efficient translation of the dendritically localized Shank1 mRNA
Katrin Studtmann et al. PLoS One. 2014.
Abstract
Local protein synthesis in dendrites enables neurons to selectively change the protein complement of individual postsynaptic sites. Though it is generally assumed that this mechanism requires tight translational control of dendritically transported mRNAs, it is unclear how translation of dendritic mRNAs is regulated. We have analyzed here translational control elements of the dendritically localized mRNA coding for the postsynaptic scaffold protein Shank1. In its 5' region, the human Shank1 mRNA exhibits two alternative translation initiation sites (AUG⁺¹ and AUG⁺²¹⁴), three canonical upstream open reading frames (uORFs1-3) and a high GC content. In reporter assays, fragments of the 5'UTR with high GC content inhibit translation, suggesting a contribution of secondary structures. uORF3 is most relevant to translation control as it overlaps with the first in frame start codon (AUG⁺¹), directing translation initiation to the second in frame start codon (AUG⁺²¹⁴). Surprisingly, our analysis points to an additional uORF initiated at a non-canonical ACG start codon. Mutation of this start site leads to an almost complete loss of translation initiation at AUG⁺¹, demonstrating that this unconventional uORF is required for Shank1 synthesis. Our data identify a novel mechanism whereby initiation at a non-canonical site allows for translation of the main Shank1 ORF despite a highly structured 5'UTR.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
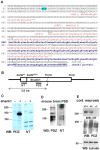
Figure 1. Alternative translational start sites in the Shank1 5′UTR.
A. Sequence of the 5′ region of the human Shank1 mRNA; seven possible AUG start codons are underlined. Translation initiation at the first three of these leads to premature termination of translation (uORFs 1–3; translation products labeled in red). AUG+1, and a cluster of three closely appositioned AUGs (collectively termed AUG+214) may both initiate translation of the full-length Shank1 ORF. Start at AUG+1 leads to an N-terminal elongation of the Shank1 protein by 70 amino acids. Amino acid sequence common to both variants is underlined. An ACG codon (nt 84–86) which becomes important later in this manuscript is indicated. B. Scheme of the Shank1 mRNA; UTRs are indicate by a line, the coding region is boxed. In frame AUGs of the main ORF, the size of a truncated expression construct of 3.8 kb, and the position of different antigenic domains (NT, PDZ) used for raising antisera are indicated. C. HEK cells were transfected with no plasmid (−) or with the truncated Shank1 expression construct (+) encompassing the first 3800 bp of the Shank1 cDNA, including the complete 5′UTR. Cell lysates were analyzed by Western blotting using an antiserum directed against the PDZ domain (left), or against the alternative N-terminal region generated by initiation at AUG+1 (NT; right). Note that the PDZ antibody recognizes two bands at approximately 130–140 kDa (calculated: 116 and 123 kDa), whereas the NT antibody recognizes only the upper one of these two bands. D. A PSD preparation derived from mouse brain was analyzed by Western blotting with PDZ and NT directed antibodies. E. Cortical neurons were prepared from wt (+/+), heterozygous (+/−) and Shank1 deficient (−/−) mice. Cells were lyzed and analyzed by Western blotting using anti-Shank1-NT (upper), anti-PDZ (middle) and anti-tubulin (lower panel) antibodies.
Figure 2. Deletion analysis of the 5′UTR of the Shank1 mRNA.
A. Schematic representation of the Shank1 5′UTR; the upper panel indicates segments with extensive secondary structure, as determined using the program mfold; AUG start codons are indicated. Deletions were introduced as indicated. B. cDNA fragments were cloned upstream of the Photinus luciferase coding sequence (control: no insert). Capped transcripts were generated, and translated in vitro using rabbit reticulocyte lysates; these were then assayed for luciferase activity to determine translation efficiency (mean +/−SD; n = 3). C. cDNA fragments were introduced into a bicistronic vector, consisting of (5′ to 3′): a CMV promoter, the cDNA fragment of interest, Photinus luciferase coding sequence, an IRES element derived from EMCV, Renilla luciferase coding sequence. Constructs were transfected into HEK cells (black) and cortical neurons (grey). Photinus and Renilla luciferase activities (Pluc; Rluc) were recorded, and translational efficiency is depicted as the Pluc/Rluc ratio (mean +/−SD; n = 5).
Figure 3. Mutational analysis of upstream open reading frames.
Individual upstream start codons of the Shank1 5′UTR were mutated to AAG, and corresponding cDNA fragments were fused to the Photinus luciferase (Pluc) coding region as described in the legend to Figure 2. For mutation of uAUG3, two bases were exchanged, changing the sequence from UGAUG (wt) to AGAUC (uAUG3mut). Translational efficiency was analyzed by in vitro translation of capped transcripts, using rabbit reticulocyte lysates (left) or in transfected HEK cells and primary cultured cortical neurons by transfecting bicistronic vectors (right), as indicated. *(***), significantly different from wt 5′UTR, p<0.05 (p<0.001); ANOVA, followed by Dunnett's test for multiple comparisons.
Figure 4. Consequences of mutation of uAUG3.
A. Fusions of the mRFP coding region with wt or uAUG3 mutant Shank1 5′UTR and in some cases also a partial Shank1 3′UTR were expressed in HEK cells; cell lysates were analyzed by Western blotting using anti-mRFP. The positions of predicted translation products initiated at AUG+1 and AUG+214 are indicated; a novel, higher molecular weight protein is indicated by a question mark. All blots were reprobed with an antibody against the product of the neomycin resistance gene (neo) encoded by the expression vector, to ensure similar transfection efficiency. B. A 3.8 kb cDNA fragment containing a large portion of the Shank1 coding region and either wt or uAUG3 mutant 5′UTR was expressed in HEK cells. Cell lysates were analyzed by Western Blotting using anti-PDZ or anti-NT antibodies. Note that the additional higher molecular weight isoform observed with the uAUG3 mutant construct is recognized by both antibodies. Blots were again reprobed with anti-neo. C. Scheme of open reading frames occurring in wt and uAUG3 mutant Shank1 5′UTR. In the wt sequence two ORFs are possible; uORF3 (indicated by brackets) is not in frame with the Shank1 main ORF. For generating uAUG3mut, two bases had to be exchanged which are depicted in grey (change from UGAUG to AGAUC); whereas uAUG3 is lost (AUG to AUC), the Shank1 open reading frame is extended in the 5′ direction due to the loss of the in frame stop codon (UGA to AGA).
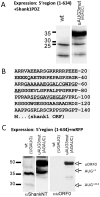
Figure 5. Identification of an N-terminally extended open reading frame.
A. cDNAs coding for WT and uAUG3 mutant Shank1 5′UTR were fused to sequence coding for the Shank1 PDZ domain. The resulting expression constructs were transfected into HEK cells, and cell lysates were analyzed by Western blotting with anti-PDZ domain antibody. B. Predicted protein translation of the 5′UTR/uAUG3 mutant mRNA in frame with the AUG+1 start codon. For identification of this protein, the uAUG3 mutant/PDZ fusion construct described in A was expressed in HEK cells, and PDZ containing proteins were affinity purified by affinity chromatography using immobilized GKAP C-terminal peptide. After SDS-PAGE analysis, the prominent band at 50 kDa (see A) was cut out and analyzed by mass spectroscopy. Non-PDZ domain peptides identified in this analysis are underlined. C. Fusions of the mRFP coding region with wt or uAUG3 mutant Shank1 5′UTR were expressed in HEK cells and analyzed by Western blotting using anti-NT and an antiserum directed against the translation product of the novel open reading frame (uORF0) depicted in B.
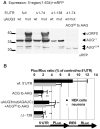
Figure 6. Identification of an unconventional start codon in the Shank1 5′UTR.
A. The mRFP coding region was fused with wt or uAUG3 mutant (AGAUC) Shank1 5′UTR; to map the translation start site, stepwise deletions of 74 and 138 bases were introduced, as well as a mutation of nucleotides 84–86 (ACG to AAG). All constructs were expressed in HEK cells and cell lysates were analyzed by Western blotting using anti-mRFP. B. Bicistronic expression vectors containing the indicated mutant versions of the Shank1 5′UTR were expressed in HEK cells and primary cultured neurons as indicated, and analyzed for the activities of the expressed luciferases as described in Figures 2 and 3. Data are presented as the percentage of the Pluc/Rluc ratio obtained with the control plasmid lacking any Shank1 5′UTR sequence. * (**), significantly different from wt 5′UTR, p<0.05 (0.01); ANOVA, followed by Dunnett's test for multiple comparisons; n = 3.
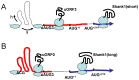
Figure 7. Model of the alternative use of upstream open reading frames in the Shank1 mRNA.
A. In the Shank1 5′UTR, the scanning 40S subunit of the ribosome encounters strong secondary structure, blocking access to translation start sites. These difficulties may be overcome either with the help of associated RNA helicase activities (h arrow) or by a shunting mechanism (s arrow). After reaching uAUG3, uORF3 (red) is translated and reinitiation at AUG+214 leads to synthesis of the short form of the mature Shank1 protein (blue). B. Alternatively, the non-canonical ACG start codon is used; uORF0 (red) is translated, allowing the 80S ribosome to proceed beyond uAUG3. Reinitiation occurs at AUG+1, leading to synthesis of the long version of Shank1 (blue).
Similar articles
- A stable upstream stem-loop structure enhances selection of the first 5'-ORF-AUG as a main start codon for translation initiation of human ACAT1 mRNA.
Yang L, Chen J, Chang CC, Yang XY, Wang ZZ, Chang TY, Li BL. Yang L, et al. Acta Biochim Biophys Sin (Shanghai). 2004 Apr;36(4):259-68. doi: 10.1093/abbs/36.4.259. Acta Biochim Biophys Sin (Shanghai). 2004. PMID: 15253151 - Negative and translation termination-dependent positive control of FLI-1 protein synthesis by conserved overlapping 5' upstream open reading frames in Fli-1 mRNA.
Sarrazin S, Starck J, Gonnet C, Doubeikovski A, Melet F, Morle F. Sarrazin S, et al. Mol Cell Biol. 2000 May;20(9):2959-69. doi: 10.1128/MCB.20.9.2959-2969.2000. Mol Cell Biol. 2000. PMID: 10757781 Free PMC article. - The upstream open reading frame mediates constitutive effects on translation of cytochrome p-450c27 from the seventh in-frame AUG codon in rat liver.
Lodhi KM, Ozdener MH, Shayiq RM. Lodhi KM, et al. J Biol Chem. 2003 Oct 17;278(42):40647-57. doi: 10.1074/jbc.M302081200. Epub 2003 Aug 7. J Biol Chem. 2003. PMID: 12909643 - The interplay between cis- and trans-acting factors drives selective mRNA translation initiation in eukaryotes.
Tidu A, Martin F. Tidu A, et al. Biochimie. 2024 Feb;217:20-30. doi: 10.1016/j.biochi.2023.09.017. Epub 2023 Sep 21. Biochimie. 2024. PMID: 37741547 Review. - A helicase links upstream ORFs and RNA structure.
Jankowsky E, Guenther UP. Jankowsky E, et al. Curr Genet. 2019 Apr;65(2):453-456. doi: 10.1007/s00294-018-0911-z. Epub 2018 Nov 27. Curr Genet. 2019. PMID: 30483885 Free PMC article. Review.
Cited by
- Alternative Splicing of the Delta-Opioid Receptor Gene Suggests Existence of New Functional Isoforms.
Piltonen M, Parisien M, Grégoire S, Chabot-Doré AJ, Jafarnejad SM, Bérubé P, Djambazian H, Sladek R, Geneau G, Willett P, Stone LS, Shabalina SA, Diatchenko L. Piltonen M, et al. Mol Neurobiol. 2019 Apr;56(4):2855-2869. doi: 10.1007/s12035-018-1253-z. Epub 2018 Jul 31. Mol Neurobiol. 2019. PMID: 30066306 - Kinases of eIF2a Switch Translation of mRNA Subset during Neuronal Plasticity.
Chesnokova E, Bal N, Kolosov P. Chesnokova E, et al. Int J Mol Sci. 2017 Oct 22;18(10):2213. doi: 10.3390/ijms18102213. Int J Mol Sci. 2017. PMID: 29065505 Free PMC article. Review. - Proteomics of the Synapse--A Quantitative Approach to Neuronal Plasticity.
Dieterich DC, Kreutz MR. Dieterich DC, et al. Mol Cell Proteomics. 2016 Feb;15(2):368-81. doi: 10.1074/mcp.R115.051482. Epub 2015 Aug 25. Mol Cell Proteomics. 2016. PMID: 26307175 Free PMC article. Review. - Tandem repeats ubiquitously flank and contribute to translation initiation sites.
Maddi AMA, Kavousi K, Arabfard M, Ohadi H, Ohadi M. Maddi AMA, et al. BMC Genom Data. 2022 Jul 27;23(1):59. doi: 10.1186/s12863-022-01075-5. BMC Genom Data. 2022. PMID: 35896982 Free PMC article. - Ribosomal selection of mRNAs with degenerate initiation triplets.
Chengguang H, Sabatini P, Brandi L, Giuliodori AM, Pon CL, Gualerzi CO. Chengguang H, et al. Nucleic Acids Res. 2017 Jul 7;45(12):7309-7325. doi: 10.1093/nar/gkx472. Nucleic Acids Res. 2017. PMID: 28575317 Free PMC article.
References
- Steward O, Schuman EM (2003) Compartmentalized synthesis and degradation of proteins in neurons. Neuron 40: 347–359. - PubMed
- Miller S, Yasuda M, Coats JK, Jones Y, Martone ME, et al. (2002) Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron 36: 507–519. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous