MicroRNA-29a modulates axon branching by targeting doublecortin in primary neurons - PubMed (original) (raw)
MicroRNA-29a modulates axon branching by targeting doublecortin in primary neurons
Hanqin Li et al. Protein Cell. 2014 Feb.
Abstract
MicroRNAs (miRNAs) are endogenously expressed small, non-coding transcripts that regulate protein expression. Substantial evidences suggest that miRNAs are enriched in central nervous system, where they are hypothesized to play pivotal roles during neural development. In the present study, we analyzed miRNAs expression in mice cerebral cortex and hippocampus at different developmental stages and found miR-29a increased dramatically at postnatal stages. In addition, we provided strong evidences that miR-29a is enriched in mature neurons both in vitro and in vivo. Further investigation demonstrated that the activation of glutamate receptors induced endogenous miR-29a level in primary neurons. Moreover, we showed that miR-29a directly regulated its target protein Doublecortin (DCX) expression, which further modulated axon branching in primary culture. Together, our results suggested that miR-29a play an important role in neuronal development of mice cerebrum.
Figures
Figure 1
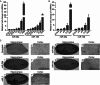
Expression of miR-29a in mice cortex and hippocampus. (A) Relative expression level of miR-29a and miR-138 (U6 is used as internal control) in cortex of different developmental stages, *, P < 0.01 compared to the E12.5 stage in each group (n = 5); (B) relative expression level of miR-29a and miR-138 in hippocampus of different developmental stages, *, P < 0.01 compared to the E18.5 stage in each group (n = 5); (C) in situ hybridization of different members of miR-29 family in hippocampus (a–e) and cortex (a′–e′) (n = 3), miR-124 is used as positive control, arrows indicate neurons with positive signal in cortex or hippocampus, scale bar = 200 μm
Figure 2
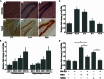
Expression of miR-29a in neurons under different circumstances. (A) Expression pattern of miR-29a (purple) in DG area of hippocampus at different postnatal stages (a–c), expression pattern of NeuN (brown) in DG area of hippocampus at different postnatal stages (d–f), granule cell layers are indicated between two yellow lines (n = 3), scale bar = 50 μm; (B) relative expression level of miR-29a in primary neuron at different cultured stages, *, P < 0.01 compared to DIV 1 in each group (n = 5); (C) relative expression level of miR-29a in primary cortical neurons at different time points after the stimulation of 10 μmol/L GSM for 15 min, *, P < 0.01 compared to control (n = 3); (D) relative expression level of miR-29a in primary cortical neurons at 2 h after indicated treatments, *, P < 0.01 (n = 3)
Figure 3
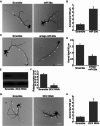
DCX is a direct target of miR-29a. (A) Seed sequence and alignment of the miR-29a binding sites in the 3′UTRs of DCX mRNAs from different species, the predicted base-pairing of miR-29a with target recognition seed sequence is shown in colored; (B) Western-blot of DCX (β-tubulin is used as internal control) in cortex (left panel) or hippocampus (right panel) at different developmental stages indicated (n = 3); (C and D) quantification of DCX protein in cortex (C) or hippocampus (D) at different developmental stages indicated (n = 3); (E) expression pattern of DCX (brown, arrows) in DG area of hippocampus at different postnatal stages, granule cell layers are between two yellow lines (n = 3), scale bar = 50 μm; (F and G) Western-blot and quantification of DCX protein in primary neurons derived from cortex and hippocampus at different time points (n = 3); (H) relative luciferase activity measured in different groups of 293T cells, *, P < 0.01 compared to control (n = 3); (I) relative level of miR-29a in primary cortical neurons transfected with miR-29a mimics, *, P < 0.01 compared to scramble (n = 3); (J and K) Western-blot and quantification of DCX protein in primary cortical neurons treated as in G, *, P < 0.01 compared to scramble (n = 3); (L) relative level of miR-29a in primary cortical neurons transfected with antago-miR-29a, *, P < 0.01 compared to scramble (n = 3); (M, N) Western-blot and quantification of DCX protein in primary cortical neurons treated as in J, *, P < 0.01 compared to scramble (n = 3)
Figure 4
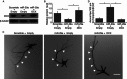
Effects of miR-29a on axon branching of primary cortical neurons. (A) Cortical neurons transfected with scramble or miR-29a mimics together with the eGFP expression plasmid were fixed 4 d after transfection and subjected to immunocytochemistry using the eGFP antibody. In all images of this type, arrowheads indicate primary axons, scale bars = 50 μm; (B) quantification of axon branching of cortical neurons treated in A, *, P < 0.01 compared to scramble (n = 20); (C) cortical neurons transfected with scramble or antago-miR-29a together with the eGFP expression plasmid were analyzed as in A, scale bars = 50 μm; (B) quantification of axon branching of cortical neurons treated in C, *, P < 0.01 compared to scramble (n = 20); (E, F) Western-blot and quantification of DCX protein in cortical neurons treated with scrambles or DCX RNAi (n = 3); (G) cortical neurons transfected with scrambles or DCX RNAi together with the eGFP expression plasmid were analyzed as in A, scale bars = 50 μm; (H) quantification of axon branching of cortical neurons treated in G, *, P < 0.01 compared to scramble (n = 20)
Figure 5
Over-expression of DCX rescues the axon branching phenotype induced by miR-29a. (A and B) Western-blot and quantification of DCX protein in cortical neurons under different treatment indicated (n = 3); (C) different groups of cortical neurons transfected together with the eGFP expression plasmid were analyzed as in Fig. 4A, scale bars = 50 μm; (D) quantification of axon branching of cortical neurons treated in C, *, P < 0.01 compared to scramble (n = 20)
Similar articles
- Stage-specific modulation of cortical neuronal development by Mmu-miR-134.
Gaughwin P, Ciesla M, Yang H, Lim B, Brundin P. Gaughwin P, et al. Cereb Cortex. 2011 Aug;21(8):1857-69. doi: 10.1093/cercor/bhq262. Epub 2011 Jan 12. Cereb Cortex. 2011. PMID: 21228099 - miR-29a Promotes the Neurite Outgrowth of Rat Neural Stem Cells by Targeting Extracellular Matrix to Repair Brain Injury.
Ma R, Wang M, Gao S, Zhu L, Yu L, Hu D, Zhu L, Huang W, Zhang W, Deng J, Pan J, He H, Gao Z, Xu J, Han X. Ma R, et al. Stem Cells Dev. 2020 May 1;29(9):599-614. doi: 10.1089/scd.2019.0174. Epub 2020 Feb 24. Stem Cells Dev. 2020. PMID: 31885334 - The microRNA-29a Modulates Serotonin 5-HT7 Receptor Expression and Its Effects on Hippocampal Neuronal Morphology.
Volpicelli F, Speranza L, Pulcrano S, De Gregorio R, Crispino M, De Sanctis C, Leopoldo M, Lacivita E, di Porzio U, Bellenchi GC, Perrone-Capano C. Volpicelli F, et al. Mol Neurobiol. 2019 Dec;56(12):8617-8627. doi: 10.1007/s12035-019-01690-x. Epub 2019 Jul 10. Mol Neurobiol. 2019. PMID: 31292861 - Identification of axon-enriched microRNAs localized to growth cones of cortical neurons.
Sasaki Y, Gross C, Xing L, Goshima Y, Bassell GJ. Sasaki Y, et al. Dev Neurobiol. 2014 Mar;74(3):397-406. doi: 10.1002/dneu.22113. Epub 2013 Sep 13. Dev Neurobiol. 2014. PMID: 23897634 Free PMC article. - The role of microRNAs in neurobiology and pathophysiology of the hippocampus.
Rashidi SK, Kalirad A, Rafie S, Behzad E, Dezfouli MA. Rashidi SK, et al. Front Mol Neurosci. 2023 Sep 4;16:1226413. doi: 10.3389/fnmol.2023.1226413. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37727513 Free PMC article. Review.
Cited by
- Neonatal anesthesia exposure impacts brain microRNAs and their associated neurodevelopmental processes.
Lin D, Liu J, Hu Z, Cottrell JE, Kass IS. Lin D, et al. Sci Rep. 2018 Jul 13;8(1):10656. doi: 10.1038/s41598-018-28874-0. Sci Rep. 2018. PMID: 30006558 Free PMC article. - Decoding the ubiquitous role of microRNAs in neurogenesis.
Nampoothiri SS, Rajanikant GK. Nampoothiri SS, et al. Mol Neurobiol. 2017 Apr;54(3):2003-2011. doi: 10.1007/s12035-016-9797-2. Epub 2016 Feb 24. Mol Neurobiol. 2017. PMID: 26910816 Review. - Systemic Analysis of miRNAs in PD Stress Condition: miR-5701 Modulates Mitochondrial-Lysosomal Cross Talk to Regulate Neuronal Death.
Prajapati P, Sripada L, Singh K, Roy M, Bhatelia K, Dalwadi P, Singh R. Prajapati P, et al. Mol Neurobiol. 2018 Jun;55(6):4689-4701. doi: 10.1007/s12035-017-0664-6. Epub 2017 Jul 14. Mol Neurobiol. 2018. PMID: 28710704 - LNC473 Regulating APAF1 IRES-Dependent Translation via Competitive Sponging miR574 and miR15b: Implications in Colorectal Cancer.
Wu H, Hu X, Li Y, Chen Q, Sun T, Qiao Y, Qin W, Wu Z, Fu B, Zhao H, Zhang R, Wei M. Wu H, et al. Mol Ther Nucleic Acids. 2020 Sep 4;21:764-779. doi: 10.1016/j.omtn.2020.07.009. Epub 2020 Jul 10. Mol Ther Nucleic Acids. 2020. PMID: 32784109 Free PMC article. - MiR-138-5p Upregulation during Neuronal Maturation Parallels with an Increase in Neuronal Survival.
Barreda-Manso MA, Soto A, Muñoz-Galdeano T, Reigada D, Nieto-Díaz M, Maza RM. Barreda-Manso MA, et al. Int J Mol Sci. 2023 Nov 20;24(22):16509. doi: 10.3390/ijms242216509. Int J Mol Sci. 2023. PMID: 38003699 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources