Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: Implication for designing novel mucosal MERS vaccines - PubMed (original) (raw)
Comparative Study
Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: Implication for designing novel mucosal MERS vaccines
Cuiqing Ma et al. Vaccine. 2014.
Abstract
Middle East respiratory syndrome (MERS) coronavirus (MERS-CoV) was originally identified in Saudi Arabia in 2012. It has caused MERS outbreaks with high mortality in the Middle East and Europe, raising a serious concern about its pandemic potential. Therefore, development of effective vaccines is crucial for preventing its further spread and future pandemic. Our previous study has shown that subcutaneous (s.c.) vaccination of a recombinant protein containing receptor-binding domain (RBD) of MERS-CoV S fused with Fc of human IgG (RBD-Fc) induced strong systemic neutralizing antibody responses in vaccinated mice. Here, we compared local and systemic immune responses induced by RBD-Fc via intranasal (i.n.) and s.c. immunization pathways. We found that i.n. vaccination of MERS-CoV RBD-Fc induced systemic humoral immune responses comparable to those induced by s.c. vaccination, including neutralizing antibodies, but more robust systemic cellular immune responses and significantly higher local mucosal immune responses in mouse lungs. This study suggests the potential of developing MERS-CoV RBD protein into an effective and safe mucosal candidate vaccine for prevention of respiratory tract infections caused by MERS-CoV.
Keywords: MERS-CoV; Mucosal immune response; Neutralizing antibody; Receptor-binding domain; Spike protein; Systemic immune response.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
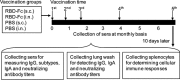
Fig. 1
Mouse immunization, sample collection and immune response detection. Four groups of mice (5 mice/group) were respectively s.c. or i.n. prime-vaccinated with MERS-CoV RBD-Fc protein plus adjuvant as the vaccine groups, or with PBS plus adjuvant as their respective controls. Mice were initially boosted twice at 21 and 42 days and further boosted at the end of the 3rd and 6th months with the same immunogens and vaccination routes, and sera were collected on a monthly basis. Ten days post-last vaccination, mouse sera and lung wash were collected to detect IgG, subtypes and IgA antibodies by ELISA, or neutralizing antibodies by neutralization assay. Mouse splenocytes collected at 10 days post-last vaccination were detected for cellular immune responses by flow cytometric analysis.
Fig. 2
Detection of total IgG antibody responses by ELISA in vaccinated mouse sera and lung wash. (A) Reactivity of serum IgG antibodies specific to MERS-CoV S1 protein. The MERS S1-specific IgG was detected using sera (1:3000) from mice before (pre-immune) and 10 days post-1st, -2nd, and -last vaccination. The data are presented as mean A450 ± standard deviation (SD) of five mice per group. (B) Ability of IgG binding to MERS-CoV S1 protein was detected using sera from 10 days post-last vaccination. The data are presented as mean A450 ± SD of five mice per group at various dilution points. MERS-CoV S1- (C) or S-RBD- (D) specific IgG endpoint titers were detected using sera from 10 days post-last vaccination. The data are presented as geometric mean titer (GMT) ± SD of five mice per group. (E) Detection of long-term antibody responses using sera collected at 0, 1, 2, 3, 4, 6 months and 10 days post-last vaccination. The data are presented as mean (IgG endpoint titers) ± SD of five mice per group. (F) Reactivity of lung wash IgG antibodies specific to MERS-CoV S1 was detected using mouse lung wash (1:1000) 10 days post-last vaccination. The data are presented as mean A450 ± SD of five mice per group.
Fig. 3
Detection of IgG subtypes by ELISA in vaccinated mouse sera. MERS-CoV S1-specific IgG1 (A), IgG2a (B) and IgG3 (C) antibodies induced by i.n. or s.c. vaccination were detected using sera from 10 days post-last vaccination. The endpoint titer of each sample was determined as the highest dilution that yielded an OD450 nm value greater than twice that of similarly diluted serum sample collected pre-immunization with an optical density of 0.15. The data are presented as GMT ± SD of five mice per group.
Fig. 4
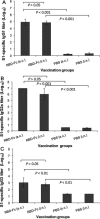
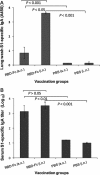
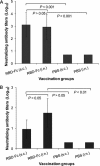
Detection of IgA antibody responses by ELISA in vaccinated mouse lung wash and sera. The MERS-CoV S1-specific IgA antibodies induced by i.n. or s.c. vaccination were detected using mouse lung wash (1:1000) (A) or serial diluted sera (B) 10 days post-last vaccination. The data from lung wash are presented as mean A450 ± SD of five mice per group. The endpoint titer of each serum sample was determined as the highest dilution that yielded an OD450 nm value greater than twice that of similarly diluted serum sample collected pre-immunization with an optical density of 0.15. The data from serum samples are presented as GMT ± SD of five mice per group.
Fig. 5
Detection of cellular immune responses by flow cytometric analysis in spleens of vaccinated mice 10 days post-last vaccination. MERS-CoV S1-specific T cell responses were detected for frequencies of IL-2- and IFN-γ-producing CD4+ splenocytes (A) and CD8+ splenocytes (B) by cell surface marker, followed by intracellular cytokine staining using flow cytometric analysis. Splenocytes from MERS-CoV RBD-Fc-vaccinated mice were incubated with recombinant MERS-CoV S1 protein (panels a and b in A and B), or SARS-CoV RBD protein (as the control) (panels e and f in A and B). Splenocytes of mice from the PBS control were also stimulated with MERS-CoV S1 (panels c and d in A and B) or SARS-CoV RBD protein (panels g and h in A and B). Frequencies of IL-2- and IFN-γ-producing cells were expressed as percentages of CD4+ or CD8+ T cells. The data in the upper left or the bottom right corner of each graph represent the frequencies of IL-2- or IFN-γ-producing CD4+ or CD8+ T cells. The samples were tested in triplicate and presented as mean ± SD (five mice per group). The numbers in bold indicate significant difference between corresponding s.c. and i.n. vaccination groups. The experiments were repeated for three times, and similar results were obtained.
Fig. 6
Detection of neutralizing antibody titers by neutralization assay in vaccinated mouse samples. Sera (A) and lung wash (1:1000 dilution in PBS during collection) (B) from i.n. or s.c. vaccination groups were collected at 10 days post-last vaccination and analyzed for neutralization of MERS-CoV infection in Vero E6 cells. Neutralizing antibody titers were expressed as the reciprocal of the highest dilution of serum that completely inhibited virus-induced CPE in at least 50% of the wells (NT50), and are presented as GMT ± SD from five mice per group.
Similar articles
- Searching for an ideal vaccine candidate among different MERS coronavirus receptor-binding fragments--the importance of immunofocusing in subunit vaccine design.
Ma C, Wang L, Tao X, Zhang N, Yang Y, Tseng CK, Li F, Zhou Y, Jiang S, Du L. Ma C, et al. Vaccine. 2014 Oct 21;32(46):6170-6176. doi: 10.1016/j.vaccine.2014.08.086. Epub 2014 Sep 19. Vaccine. 2014. PMID: 25240756 Free PMC article. - Recombinant Receptor-Binding Domains of Multiple Middle East Respiratory Syndrome Coronaviruses (MERS-CoVs) Induce Cross-Neutralizing Antibodies against Divergent Human and Camel MERS-CoVs and Antibody Escape Mutants.
Tai W, Wang Y, Fett CA, Zhao G, Li F, Perlman S, Jiang S, Zhou Y, Du L. Tai W, et al. J Virol. 2016 Dec 16;91(1):e01651-16. doi: 10.1128/JVI.01651-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795425 Free PMC article. - A Novel Bacterium-Like Particle Vaccine Displaying the MERS-CoV Receptor-Binding Domain Induces Specific Mucosal and Systemic Immune Responses in Mice.
Li E, Chi H, Huang P, Yan F, Zhang Y, Liu C, Wang Z, Li G, Zhang S, Mo R, Jin H, Wang H, Feng N, Wang J, Bi Y, Wang T, Sun W, Gao Y, Zhao Y, Yang S, Xia X. Li E, et al. Viruses. 2019 Aug 29;11(9):799. doi: 10.3390/v11090799. Viruses. 2019. PMID: 31470645 Free PMC article. - Prospects for a MERS-CoV spike vaccine.
Zhou Y, Jiang S, Du L. Zhou Y, et al. Expert Rev Vaccines. 2018 Aug;17(8):677-686. doi: 10.1080/14760584.2018.1506702. Epub 2018 Aug 9. Expert Rev Vaccines. 2018. PMID: 30058403 Free PMC article. Review. - Advances in MERS-CoV Vaccines and Therapeutics Based on the Receptor-Binding Domain.
Zhou Y, Yang Y, Huang J, Jiang S, Du L. Zhou Y, et al. Viruses. 2019 Jan 14;11(1):60. doi: 10.3390/v11010060. Viruses. 2019. PMID: 30646569 Free PMC article. Review.
Cited by
- Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus.
Zhang N, Channappanavar R, Ma C, Wang L, Tang J, Garron T, Tao X, Tasneem S, Lu L, Tseng CT, Zhou Y, Perlman S, Jiang S, Du L. Zhang N, et al. Cell Mol Immunol. 2016 Mar;13(2):180-90. doi: 10.1038/cmi.2015.03. Epub 2015 Feb 2. Cell Mol Immunol. 2016. PMID: 25640653 Free PMC article. - Intranasal and oral vaccination with protein-based antigens: advantages, challenges and formulation strategies.
Wang S, Liu H, Zhang X, Qian F. Wang S, et al. Protein Cell. 2015 Jul;6(7):480-503. doi: 10.1007/s13238-015-0164-2. Epub 2015 May 6. Protein Cell. 2015. PMID: 25944045 Free PMC article. Review. - Broad-Spectrum Anti-coronavirus Vaccines and Therapeutics to Combat the Current COVID-19 Pandemic and Future Coronavirus Disease Outbreaks.
Cao M, Su X, Jiang S. Cao M, et al. Stem Cell Reports. 2021 Mar 9;16(3):398-411. doi: 10.1016/j.stemcr.2020.12.010. Stem Cell Reports. 2021. PMID: 33691145 Free PMC article. Review. - Molecular Characteristics, Functions, and Related Pathogenicity of MERS-CoV Proteins.
Li YH, Hu CY, Wu NP, Yao HP, Li LJ. Li YH, et al. Engineering (Beijing). 2019 Oct;5(5):940-947. doi: 10.1016/j.eng.2018.11.035. Epub 2019 Jul 17. Engineering (Beijing). 2019. PMID: 32288963 Free PMC article. Review. - Viral vectored vaccines: design, development, preventive and therapeutic applications in human diseases.
Wang S, Liang B, Wang W, Li L, Feng N, Zhao Y, Wang T, Yan F, Yang S, Xia X. Wang S, et al. Signal Transduct Target Ther. 2023 Apr 7;8(1):149. doi: 10.1038/s41392-023-01408-5. Signal Transduct Target Ther. 2023. PMID: 37029123 Free PMC article. Review.
References
- Zaki A.M., van B.S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. - PubMed
- Memish Z.A., Zumla A.I., Assiri A. Middle East respiratory syndrome coronavirus infections in health care workers. N Engl J Med. 2013;369:884–886. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources