Dopamine mediates vagal modulation of the immune system by electroacupuncture - PubMed (original) (raw)
Dopamine mediates vagal modulation of the immune system by electroacupuncture
Rafael Torres-Rosas et al. Nat Med. 2014 Mar.
Abstract
Previous anti-inflammatory strategies against sepsis, a leading cause of death in hospitals, had limited efficacy in clinical trials, in part because they targeted single cytokines and the experimental models failed to mimic clinical settings. Neuronal networks represent physiological mechanisms, selected by evolution to control inflammation, that can be exploited for the treatment of inflammatory and infectious disorders. Here, we report that sciatic nerve activation with electroacupuncture controls systemic inflammation and rescues mice from polymicrobial peritonitis. Electroacupuncture at the sciatic nerve controls systemic inflammation by inducing vagal activation of aromatic L-amino acid decarboxylase, leading to the production of dopamine in the adrenal medulla. Experimental models with adrenolectomized mice mimic clinical adrenal insufficiency, increase the susceptibility to sepsis and prevent the anti-inflammatory effects of electroacupuncture. Dopamine inhibits cytokine production via dopamine type 1 (D1) receptors. D1 receptor agonists suppress systemic inflammation and rescue mice with adrenal insufficiency from polymicrobial peritonitis. Our results suggest a new anti-inflammatory mechanism mediated by the sciatic and vagus nerves that modulates the production of catecholamines in the adrenal glands. From a pharmacological perspective, the effects of selective dopamine agonists mimic the anti-inflammatory effects of electroacupuncture and can provide therapeutic advantages to control inflammation in infectious and inflammatory disorders.
Figures
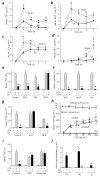
Figure 1. Electroacupuncture controls systemic inflammation in sepsis via the sciatic, the vagus nerves, and catecholamines from the adrenal glands
(a–d) Adult C57BL/6 male mice were treated with LPS and sham or electroacupuncture (EA). Serum cytokines levels of TNF (a), MCP1 (b), IL6 (c) and INFγ (d) were analyzed at the indicated time points post-LPS. (e) Mice underwent sham (Control) or surgical neurectomy of the common peroneal (PSX), tibial (TSX), or sciatic nerve (SSX) 72 h before the LPS challenge and the sham or electroacupuncture (EA). *P<0.01 vs LPS (_n_=3/group; Student’s t–test) (f) Mice underwent sham or sciatic nerve stimulation (ScNS) for 15 min before the LPS challenge. (g) Mice underwent sham (Control), subdiaphragmatic vagotomy (sVX) 72 h, or splenectomy (SPX) 3 days before the LPS challenge and the subsequent sham or electroacupuncture (EA). (h) Blood from untreated animals (Blood) was supplemented with 50% serum from donor animals that underwent sham (Control) or electroacupuncture in sham (EA) or adrenalectomized (ADX/EA) mice. Upper panels represent cell survival as determined by MTT assay. (t-test EA vs. ADX/EA). (i) Mice underwent control, surgical adrenalectomy (ADX), or treatment with Reserpine before electroacupuncture (EA). (j) Dopamine (DA), norepinephrine (NE) or epinephrine (E) were analyzed in the serum of control or adrenalectomized (ADX) mice with sham or electroacupuncture (EA). TNF was analyzed at 90 min in the serum or 180 min in the conditioned media post-LPS. *P<0.01 (_n_=4/group; One-way ANOVA with Bonferroni’s corrections).
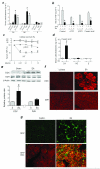
Figure 2. Electroacupuncture induces the expression of DOPA decarboxylase and dopamine
(a) Catecholamines were analyzed in the serum C57BL/6 mice with electroacupuncture in animals with subdiaphragmatic vagotomy (EA+sVX), vagus nerve stimulation (VNS), or electroacupuncture in α_7nAChR_-knockout (EA-7KO) mice. ND=Not Detected (b) Serum TNF levels were analyzed in C57BL/6 mice (Control), α_7nAChR_-knockout (α7KO) mice, _β2adrenoceptor_-knockout (β2KO) mice, or C57BL/6 mice treated with fusaric acid (40mg kg−1; i.p.) and electroacupuncture (EA). (c) Blood from wild-type (WT) or _β2adrenoceptor_-knockout (β2KO) mice was treated in vitro with LPS and the indicated concentrations of norepinephrine (NE). (d) Mice received fusaric acid (40mg kg−1; i.p.), and sham or electroacupuncture (EA) and serum levels of catecholamines were analyzed after 15 min post-EA. (e) The adrenal glands from adult C57BL/6 male mice that received sham (S) or electroacupuncture (EA) were collected and analyzed by Western-blot for DOPA decarboxylase (DDC) and Dopamine β–hydroxylase (DβH) expression. β–Actin was used to normalize protein loading. The graph depicts the relative values of the densitometry of the experimental samples as compared with the control. In all experiments *P<0.01 vs Sham (S) (_n_=3/group; Student’s t-test). (f) The adrenal glands from mice with sham or electroacupuncture (EA) were analyzed for immunostaining for DOPA decarboxylase (DDC) or Dopamine β–hydroxylase (DβH). (g) The adrenal glands from mice subjected to control or electroacupuncture (EA) were stained for phosphorylated neurofilaments (NF-P; green) or double stained for phosphorylated neurofilaments (NF-P; green) and DOPA decarboxylase (DDC; red). The figures are representative of three different experiments. Bars represent 100 μm.
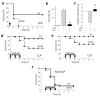
Figure 3. Electroacupuncture “rescues” mice from established polymicrobial peritonitis
(a-c) Adult C57BL/6 male mice underwent sham or electroacupuncture (EA) started right after the endotoxin challenge (LPS; 12 mg kg−1; i.p.). (a) Survival was represented in a Kaplan-Meier analysis (_n_=20/group, *P<0.01 Survival Logrank test vs. control). (b) Morbidity and (c) weight modifications were represented in mean ± SE. *P<0.01 (_n_=6/group; One-way ANOVA with Bonferroni’s corrections). (d-e) All mice underwent CLP and sham or electroacupuncture (EA) started (d) 15 min before or (e) 24 h after the CLP. In addition to the first treatment, all animals received sham or electroacupuncture for 15 min once a day for the next two days. Survival was represented in a Kaplan-Meier analysis (_n_=15/group, *P<0.05 Survival Logrank test vs. control). (f) Mice underwent surgical adrenalectomy (ADX) before CLP and subsequent sham or electroacupuncture (EA, given once a day for three days). Survival is represented in a Kaplan-Meier analysis (_n_=20/group, *P<0.05 Survival Logrank test vs. control).
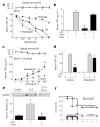
Figure 4. D1R-agonist “rescues” mice from established polymicrobial peritonitis with adrenal insufficiency
(a) Blood was treated with Dopamine (DA), Fenoldopam (Fenol) or Pergolide (Perg) 30 min before the LPS challenge. TNF levels were analyzed at 3 h post-LPS. Upper panels represent cell survival as determined by MTT assay. (b) Mice received Fenoldopam (10 mg kg−1 per dose; i.p.) or Pergolide (10 mg kg−1 per dose; i.p.) before the LPS challenge. Serum TNF levels were analyzed at 90 min post-LPS. *P<0.01 vs LPS (_n_=3/group; One-way ANOVA with Bonferroni’s corrections). (c) Untreated blood (Blood) was given vehicle (control) or Butaclamol 30 min before supplemented with 50% mice serum with electroacupuncture (EA Serum) and treated with LPS. TNF concentrations were analyzed 3 h later. (d) Mice were treated with butaclamol (12mg kg−1 per dose; i.p.) 60 min before the LPS challenge, and received sham or electroacupuncture (EA). Serum TNF levels were analyzed 90 min post-LPS. (e) Mice underwent sham (control) or CLP with vehicle or Fenoldopam (Fenol). Serum HMGB1 levels were analyzed at 24 h post-CLP. The graph below depicts the relative percent of serum HMGB1 levels as compared to CLP *P<0.01 (_n_=3/group; One-way ANOVA with Bonferroni’s corrections). (f) Mice underwent adrenalectomy (ADX) before CLP. Arrows represent the three doses of vehicle (control) or Fenoldopam (10mg kg−1 per dose; i.p.) started 1 day after the CLP, and given every 12 h for three days. Survival was recorded and represented in a Kaplan-Meier analysis (_n_=20/group, *P<0.01 Survival Logrank test vs. control).
Comment in
- Regulating innate immunity with dopamine and electroacupuncture.
Chavan SS, Tracey KJ. Chavan SS, et al. Nat Med. 2014 Mar;20(3):239-41. doi: 10.1038/nm.3501. Nat Med. 2014. PMID: 24603793 No abstract available.
Similar articles
- Sciatic-Vagal Nerve Stimulation by Electroacupuncture Alleviates Inflammatory Arthritis in Lyme Disease-Susceptible C3H Mice.
Akoolo L, Djokic V, Rocha SC, Ulloa L, Parveen N. Akoolo L, et al. Front Immunol. 2022 Jul 18;13:930287. doi: 10.3389/fimmu.2022.930287. eCollection 2022. Front Immunol. 2022. PMID: 35924250 Free PMC article. - Regulating innate immunity with dopamine and electroacupuncture.
Chavan SS, Tracey KJ. Chavan SS, et al. Nat Med. 2014 Mar;20(3):239-41. doi: 10.1038/nm.3501. Nat Med. 2014. PMID: 24603793 No abstract available. - Glucose Activates Vagal Control of Hyperglycemia and Inflammation in Fasted Mice.
Joseph B, Shimojo G, Li Z, Thompson-Bonilla MDR, Shah R, Kanashiro A, Salgado HC, Ulloa L. Joseph B, et al. Sci Rep. 2019 Jan 30;9(1):1012. doi: 10.1038/s41598-018-36298-z. Sci Rep. 2019. PMID: 30700738 Free PMC article. - The vagus nerve and the inflammatory reflex: wandering on a new treatment paradigm for systemic inflammation and sepsis.
Huston JM. Huston JM. Surg Infect (Larchmt). 2012 Aug;13(4):187-93. doi: 10.1089/sur.2012.126. Epub 2012 Aug 22. Surg Infect (Larchmt). 2012. PMID: 22913335 Review. - Vagal Modulation of the Inflammatory Response in Sepsis.
Wang DW, Yin YM, Yao YM. Wang DW, et al. Int Rev Immunol. 2016 Sep 2;35(5):415-433. doi: 10.3109/08830185.2015.1127369. Epub 2016 Apr 29. Int Rev Immunol. 2016. PMID: 27128144 Review.
Cited by
- Chemokinergic and Dopaminergic Signalling Collaborates through the Heteromer Formed by CCR9 and Dopamine Receptor D5 Increasing the Migratory Speed of Effector CD4+ T-Cells to Infiltrate the Colonic Mucosa.
Campos J, Osorio-Barrios F, Villanelo F, Gutierrez-Maldonado SE, Vargas P, Pérez-Acle T, Pacheco R. Campos J, et al. Int J Mol Sci. 2024 Sep 18;25(18):10022. doi: 10.3390/ijms251810022. Int J Mol Sci. 2024. PMID: 39337509 Free PMC article. - Acupuncture versus Various Control Treatments in the Treatment of Migraine: A Review of Randomized Controlled Trials from the Past 10 Years.
Ni X, Dong L, Tian T, Liu L, Li X, Li F, Zhao L. Ni X, et al. J Pain Res. 2020 Aug 12;13:2033-2064. doi: 10.2147/JPR.S259390. eCollection 2020. J Pain Res. 2020. PMID: 32884332 Free PMC article. Review. - Electroacupuncture May Inhibit Oxidative Stress of Premature Ovarian Failure Mice by Regulating Intestinal Microbiota.
Geng Z, Nie X, Ling L, Li B, Liu P, Yuan L, Zhang K, Liu T, Zhang B. Geng Z, et al. Oxid Med Cell Longev. 2022 Aug 30;2022:4362317. doi: 10.1155/2022/4362317. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36082082 Free PMC article. - Active Acupoints Differ from Inactive Acupoints in Modulating Key Plasmatic Metabolites of Hypertension: A Targeted Metabolomics Study.
Yang M, Yu Z, Chen X, Guo Z, Deng S, Chen L, Wu Q, Liang F. Yang M, et al. Sci Rep. 2018 Dec 13;8(1):17824. doi: 10.1038/s41598-018-36199-1. Sci Rep. 2018. PMID: 30546033 Free PMC article. Clinical Trial. - Electroacupuncture for the Prevention of Postoperative Cognitive Dysfunction Among Older Adults Undergoing Hip and Knee Arthroplasty: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.
Ou L, Shen Z, Zhang T, Chen Z, Zhang L, Xu D, Kong D, Qi Q, Huang Y, Huang W, Meng Y. Ou L, et al. Front Med (Lausanne). 2022 Jan 4;8:778474. doi: 10.3389/fmed.2021.778474. eCollection 2021. Front Med (Lausanne). 2022. PMID: 35059414 Free PMC article.
References
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. - PubMed
- Ulloa L, Tracey KJ. The "cytokine profile": a code for sepsis. Trends Mol Med. 2005;11:56–63. - PubMed
- Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–684. - PubMed
- Annane D. Adrenal insufficiency in sepsis. Curr Pharm Des. 2008;14:1882–1886. - PubMed
- Tracey KJ, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases