Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells - PubMed (original) (raw)
Review
Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells
Anna Park et al. World J Stem Cells. 2014.
Abstract
Adipose tissue is a major metabolic organ, and it has been traditionally classified as either white adipose tissue (WAT) or brown adipose tissue (BAT). WAT and BAT are characterized by different anatomical locations, morphological structures, functions, and regulations. WAT and BAT are both involved in energy balance. WAT is mainly involved in the storage and mobilization of energy in the form of triglycerides, whereas BAT specializes in dissipating energy as heat during cold- or diet-induced thermogenesis. Recently, brown-like adipocytes were discovered in WAT. These brown-like adipocytes that appear in WAT are called beige or brite adipocytes. Interestingly, these beige/brite cells resemble white fat cells in the basal state, but they respond to thermogenic stimuli with increased levels of thermogenic genes and increased respiration rates. In addition, beige/brite cells have a gene expression pattern distinct from that of either white or brown fat cells. The current epidemic of obesity has increased the interest in studying adipocyte formation (adipogenesis), especially in beige/brite cells. This review summarizes the developmental process of adipose tissues that originate from the mesenchymal stem cells and the features of these three different types of adipocytes.
Keywords: Adipogenesis; Beige/brite adipocytes; Brown adipocytes; Browning; Mesenchymal stem cells; Thermogenesis; White adipocytes.
Figures
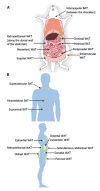
Figure 1
Locations of adipose tissue depots in a mouse (A) and an adult human (B). A: Subcutaneous (inguinal and intramuscular), visceral (mesenteric, omental, perigonadal and retroperitoneal) and brown (interscapular and perirenal) adipose tissue depots are shown in a mouse model; B: Subcutaneous (abdominal, femoral and gluteal), visceral (epicardial, gonadal, mesenteric, omental and retroperitoneal) and brown (paravertebral, supraclavicular and suprarenal) adipose tissue depots are shown in a human model. WAT: White adipose tissue; BAT: Brown adipose tissue.
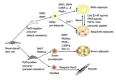
Figure 2
Differentiation into white, beige or brown adipocytes. Previously, white and brown adipocytes were thought to be derived from the same precursor cell. However, recent studies demonstrated that brown fat shares a progenitor cell (Myf5+) with skeletal muscle and not with white adipocytes. The Myf5+ precursors are induced to transform into mature brown adipocytes by bone morphogenetic protein 7 (BMP7), peroxisome proliferator-activated receptor-γ (PPAR-γ) and CCAAT/enhancer-binding proteins (C/EBPs) in cooperation with the transcriptional co-regulator PR domain-containing 16 (PRDM16) and PGC-1α. White adipocytes can also be transformed to brown-like adipocytes, called beige/brite adipocytes, by cold exposure, a β-adrenergic agonist or a PPAR-γ agonist. AR: adrenergic receptor; FGF21: Fibroblast growth factor 21; PGC-1α: Peroxisome proliferator activated receptor gamma coactivator 1 alpha.
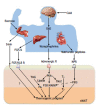
Figure 3
Key regulators of the browning process and their action mechanisms. Browning is induced by sympathetic nervous system (SNS)-independent or SNS-dependent signals. These signals sometimes synergistically or competitively influence the activation of browning of subcutaneous white adipose tissue (sWAT). Irisin is a newly discovered myokine and is released by skeletal muscle during exercise. Irisin induces the browning process of sWAT. Fibroblast growth factor-21 (FGF21), a hormonal factor from the liver, directly activates the thermogenic process via interaction with the FGF receptor/β-Klotho (KLB) complex. The norepinephrine secreted by the SNS in response to thermogenic stimuli induces the activation of adrenergic receptor(s). The adrenergic receptor-mediated signal increases the level of intracellular cAMP and activates cAMP-dependent protein kinase A (PKA). Subsequently, PKA activates p38 MAP kinase (p38 MAPK) and 5’-deiodinase 2 (Dio2), which catalyzes the conversion of thyroxine (T4) into the active form 3,5,3’-tri-iodothyronine (T3). Then, it ultimately induces the gene process for thermogenic activation. Natriuretic peptides (NPs) originating from the heart activate the thermogenic process through binding to the NP receptor, activation of protein kinase G (PKG) and the subsequent activation of p38 MAPK and NPR. NPR: Natriuretic peptides receptor; TAG: Triacylglycerol; FA: Fatty acid.
Similar articles
- White, brown, beige/brite: different adipose cells for different functions?
Giralt M, Villarroya F. Giralt M, et al. Endocrinology. 2013 Sep;154(9):2992-3000. doi: 10.1210/en.2013-1403. Epub 2013 Jun 19. Endocrinology. 2013. PMID: 23782940 Review. - Overexpression of Adiponectin Receptor 1 Inhibits Brown and Beige Adipose Tissue Activity in Mice.
Chen YJ, Lin CW, Peng YJ, Huang CW, Chien YS, Huang TH, Liao PX, Yang WY, Wang MH, Mersmann HJ, Wu SC, Chuang TY, Lin YY, Kuo WH, Ding ST. Chen YJ, et al. Int J Mol Sci. 2021 Jan 18;22(2):906. doi: 10.3390/ijms22020906. Int J Mol Sci. 2021. PMID: 33477525 Free PMC article. - Thermogenic brown and beige/brite adipogenesis in humans.
Cereijo R, Giralt M, Villarroya F. Cereijo R, et al. Ann Med. 2015 Mar;47(2):169-77. doi: 10.3109/07853890.2014.952328. Epub 2014 Sep 18. Ann Med. 2015. PMID: 25230914 Review. - Brite/beige fat and UCP1 - is it thermogenesis?
Keipert S, Jastroch M. Keipert S, et al. Biochim Biophys Acta. 2014 Jul;1837(7):1075-82. doi: 10.1016/j.bbabio.2014.02.008. Epub 2014 Feb 14. Biochim Biophys Acta. 2014. PMID: 24530356 - Role of Ginsenosides in Browning of White Adipose Tissue to Combat Obesity: A Narrative Review on Molecular Mechanism.
Pu J, Akter R, Rupa EJ, Awais M, Mathiyalagan R, Han Y, Kang J, Yang DC, Kang SC. Pu J, et al. Arch Med Res. 2022 Apr;53(3):231-239. doi: 10.1016/j.arcmed.2021.11.003. Epub 2021 Dec 11. Arch Med Res. 2022. PMID: 34906389 Review.
Cited by
- Selection of Suitable Reference Genes for Quantitative Real-Time PCR Normalization in Three Types of Rat Adipose Tissue.
Zhang WX, Fan J, Ma J, Rao YS, Zhang L, Yan YE. Zhang WX, et al. Int J Mol Sci. 2016 Jun 22;17(6):968. doi: 10.3390/ijms17060968. Int J Mol Sci. 2016. PMID: 27338366 Free PMC article. - Asprosin contributes to pathogenesis of obesity by adipocyte mitophagy induction to inhibit white adipose browning in mice.
Chen S, Yuan W, Huang Q, Xiong X, Wang C, Zeng W, Wang L, Huang Y, Liu Y, Wang Y, Huang Q. Chen S, et al. Int J Obes (Lond). 2024 Jul;48(7):913-922. doi: 10.1038/s41366-024-01495-6. Epub 2024 Feb 19. Int J Obes (Lond). 2024. PMID: 38374247 - Anti-obesity and metabolic benefits of metformin: Comparison of different delivery routes.
Abbasi M, Fan Z, Dawson JA, Wang S. Abbasi M, et al. J Drug Deliv Sci Technol. 2024 Jan;91:105110. doi: 10.1016/j.jddst.2023.105110. Epub 2023 Nov 15. J Drug Deliv Sci Technol. 2024. PMID: 38188941 Free PMC article. - Adipose tissue aging is regulated by an altered immune system.
Zhang YX, Ou MY, Yang ZH, Sun Y, Li QF, Zhou SB. Zhang YX, et al. Front Immunol. 2023 Feb 17;14:1125395. doi: 10.3389/fimmu.2023.1125395. eCollection 2023. Front Immunol. 2023. PMID: 36875140 Free PMC article. Review. - Betulinic acid decreases lipid accumulation in adipogenesis-induced human mesenchymal stem cells with upregulation of PGC-1α and UCP-1 and post-transcriptional downregulation of adiponectin and leptin secretion.
Senamontree S, Lakthan T, Charoenpanich P, Chanchao C, Charoenpanich A. Senamontree S, et al. PeerJ. 2021 Oct 14;9:e12321. doi: 10.7717/peerj.12321. eCollection 2021. PeerJ. 2021. PMID: 34721992 Free PMC article.
References
- Lean ME. Brown adipose tissue in humans. Proc Nutr Soc. 1989;48:243–256. - PubMed
- Enerbäck S. Human brown adipose tissue. Cell Metab. 2010;11:248–252. - PubMed
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources