The agricultural antibiotic carbadox induces phage-mediated gene transfer in Salmonella - PubMed (original) (raw)
The agricultural antibiotic carbadox induces phage-mediated gene transfer in Salmonella
Bradley L Bearson et al. Front Microbiol. 2014.
Abstract
Antibiotics are used for disease therapeutic or preventative effects in humans and animals, as well as for enhanced feed conversion efficiency in livestock. Antibiotics can also cause undesirable effects in microbial populations, including selection for antibiotic resistance, enhanced pathogen invasion, and stimulation of horizontal gene transfer. Carbadox is a veterinary antibiotic used in the US during the starter phase of swine production for improved feed efficiency and control of swine dysentery and bacterial swine enteritis. Carbadox has been shown in vitro to induce phage-encoded Shiga toxin in Shiga toxin-producing Escherichia coli (STEC) and a phage-like element transferring antibiotic resistance genes in Brachyspira hyodysenteriae, but the effect of carbadox on prophages in other bacteria is unknown. This study examined carbadox exposure on prophage induction and genetic transfer in Salmonella enterica serovar Typhimurium, a human foodborne pathogen that frequently colonizes swine without causing disease. S. Typhimurium LT2 exposed to carbadox induced prophage production, resulting in bacterial cell lysis and release of virions that were visible by electron microscopy. Carbadox induction of phage-mediated gene transfer was confirmed by monitoring the transduction of a sodCIII::neo cassette in the Fels-1 prophage from LT2 to a recipient Salmonella strain. Furthermore, carbadox frequently induced generalized transducing phages in multidrug-resistant phage type DT104 and DT120 isolates, resulting in the transfer of chromosomal and plasmid DNA that included antibiotic resistance genes. Our research indicates that exposure of Salmonella to carbadox induces prophages that can transfer virulence and antibiotic resistance genes to susceptible bacterial hosts. Carbadox-induced, phage-mediated gene transfer could serve as a contributing factor in bacterial evolution during animal production, with prophages being a reservoir for bacterial fitness genes in the environment.
Keywords: Salmonella; antibiotic; bacteriophage; carbadox; gene transfer; prophage; transduction.
Figures
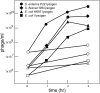
Figure 1
Carbadox induction of Enterobacteriaeae prophages. Bacterial strains lysogenized with P22 (UB-1790), λ (UB-1703), HK97 (UB-1704), and Sf6 (UB-1496) were grown in LB broth at 37°C with shaking. At a density of 1 × 108 bacterial cells/ml, carbadox was added to a final concentration of 0.5 μg/ml. Phage lysates were obtained by shaking with chloroform at the indicated times after carbadox addition and titered on a permissive host. Open symbols indicate cultures with no added carbadox and closed symbols indicate cultures with carbadox added. The different induced lysogens are indicated in the figure insert.
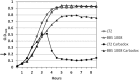
Figure 2
Carbadox exposure of wild-type S. Typhimurium LT2 results in bacterial cell lysis. S. Typhimurium strains (LT2 and BBS 1008) were grown in LB glucose medium at 37°C with shaking. At OD600 = 0.2 (arrow), carbadox (2.5 μg/ml) was added to cultures indicated by the closed symbols. The open symbols indicate control cultures without carbadox.
Similar articles
- Fluoroquinolone induction of phage-mediated gene transfer in multidrug-resistant Salmonella.
Bearson BL, Brunelle BW. Bearson BL, et al. Int J Antimicrob Agents. 2015 Aug;46(2):201-4. doi: 10.1016/j.ijantimicag.2015.04.008. Epub 2015 May 28. Int J Antimicrob Agents. 2015. PMID: 26078016 - The In-Feed Antibiotic Carbadox Induces Phage Gene Transcription in the Swine Gut Microbiome.
Johnson TA, Looft T, Severin AJ, Bayles DO, Nasko DJ, Wommack KE, Howe A, Allen HK. Johnson TA, et al. mBio. 2017 Aug 8;8(4):e00709-17. doi: 10.1128/mBio.00709-17. mBio. 2017. PMID: 28790203 Free PMC article. - Antibiotics in feed induce prophages in swine fecal microbiomes.
Allen HK, Looft T, Bayles DO, Humphrey S, Levine UY, Alt D, Stanton TB. Allen HK, et al. mBio. 2011 Nov 29;2(6):e00260-11. doi: 10.1128/mBio.00260-11. Print 2011. mBio. 2011. PMID: 22128350 Free PMC article. - Altruism of Shiga toxin-producing Escherichia coli: recent hypothesis versus experimental results.
Loś JM, Loś M, Węgrzyn A, Węgrzyn G. Loś JM, et al. Front Cell Infect Microbiol. 2013 Jan 4;2:166. doi: 10.3389/fcimb.2012.00166. eCollection 2012. Front Cell Infect Microbiol. 2013. PMID: 23316482 Free PMC article. Review. - Bacteriophages Contribute to the Spread of Antibiotic Resistance Genes among Foodborne Pathogens of the Enterobacteriaceae Family - A Review.
Colavecchio A, Cadieux B, Lo A, Goodridge LD. Colavecchio A, et al. Front Microbiol. 2017 Jun 20;8:1108. doi: 10.3389/fmicb.2017.01108. eCollection 2017. Front Microbiol. 2017. PMID: 28676794 Free PMC article. Review.
Cited by
- Bacteriophage P22 SieA-mediated superinfection exclusion.
Leavitt JC, Woodbury BM, Gilcrease EB, Bridges CM, Teschke CM, Casjens SR. Leavitt JC, et al. mBio. 2024 Feb 14;15(2):e0216923. doi: 10.1128/mbio.02169-23. Epub 2024 Jan 18. mBio. 2024. PMID: 38236051 Free PMC article. - Cross-Domain and Viral Interactions in the Microbiome.
Rowan-Nash AD, Korry BJ, Mylonakis E, Belenky P. Rowan-Nash AD, et al. Microbiol Mol Biol Rev. 2019 Jan 9;83(1):e00044-18. doi: 10.1128/MMBR.00044-18. Print 2019 Mar. Microbiol Mol Biol Rev. 2019. PMID: 30626617 Free PMC article. Review. - Virus ecology and disturbances: impact of environmental disruption on the viruses of microorganisms.
Allen HK, Abedon ST. Allen HK, et al. Front Microbiol. 2014 Dec 12;5:700. doi: 10.3389/fmicb.2014.00700. eCollection 2014. Front Microbiol. 2014. PMID: 25566216 Free PMC article. No abstract available. - DNA Gyrase Inhibitors Increase the Frequency of Bacteriophage-like RcGTA-Mediated Gene Transfer in Rhodobacter capsulatus.
Bernelot-Moens R, Beatty JT. Bernelot-Moens R, et al. Genes (Basel). 2022 Nov 9;13(11):2071. doi: 10.3390/genes13112071. Genes (Basel). 2022. PMID: 36360308 Free PMC article. - Carbadox has both temporary and lasting effects on the swine gut microbiota.
Looft T, Allen HK, Casey TA, Alt DP, Stanton TB. Looft T, et al. Front Microbiol. 2014 Jun 10;5:276. doi: 10.3389/fmicb.2014.00276. eCollection 2014. Front Microbiol. 2014. PMID: 24959163 Free PMC article.
References
- Bearson B. L., Bearson S. M., Uthe J. J., Dowd S. E., Houghton J. O., Lee I., et al. (2008). Iron regulated genes of Salmonella enterica serovar Typhimurium in response to norepinephrine and the requirement of fepDGC for norepinephrine-enhanced growth. Microbes Infect. 10, 807–816 10.1016/j.micinf.2008.04.011 - DOI - PubMed
- Boyd D., Peters G. A., Cloeckaert A., Boumedine K. S., Chaslus-Dancla E., Imberechts H., et al. (2001). Complete nucleotide sequence of a 43-kilobase genomic island associated with the multidrug resistance region of Salmonella enterica serovar Typhimurium DT104 and its identification in phage type DT120 and serovar Agona. J. Bacteriol. 183, 5725–5732 10.1128/JB.183.19.5725-5732.2001 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources