Composite three-dimensional woven scaffolds with interpenetrating network hydrogels to create functional synthetic articular cartilage - PubMed (original) (raw)
Composite three-dimensional woven scaffolds with interpenetrating network hydrogels to create functional synthetic articular cartilage
I-Chien Liao et al. Adv Funct Mater. 2013.
Abstract
The development of synthetic biomaterials that possess mechanical properties that mimic those of native tissues remains an important challenge to the field of materials. In particular, articular cartilage is a complex nonlinear, viscoelastic, and anisotropic material that exhibits a very low coefficient of friction, allowing it to withstand millions of cycles of joint loading over decades of wear. Here we show that a three-dimensionally woven fiber scaffold that is infiltrated with an interpenetrating network hydrogel can provide a functional biomaterial that provides the load-bearing and tribological properties of native cartilage. An interpenetrating dual-network "tough-gel" consisting of alginate and polyacrylamide was infused into a porous three-dimensionally woven poly(ε-caprolactone) fiber scaffold, providing a versatile fiber-reinforced composite structure as a potential acellular or cell-based replacement for cartilage repair.
Keywords: 3-D weaving; IPN; Osteoarthritis; interpenetrating network hydrogels; scaffold; synthetic articular cartilage; tissue engineering.
Figures
Figure 1
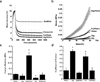
Compressive and frictional properties of IPN hydrogels. PAAm = polyacrylamide, Alg = alginate, Fib = fibrin, Alg/PAAm = alginate/polyacrylamide & Fib/PAAm = fibrin/polyacrylamide) (A) PCL scaffold alone, PCL/Alg/PAAm-IPN composite, cartilage, and Alg/PAAm strain recovery following 10% strain, (B) Stress-strain behavior of the hydrogels during compression, (C) Young’s modulus of the hydrogels calculated from unconfined compression studies. * P < 0.05 for Alg/PAAm vs. PAAm, Alg, Fib and Fib/PAAm. + P < 0.05 for Fib/PAAm vs. PAAm and Fib. (D) Coefficient of friction of the hydrogels determined using a rheometer. * P < 0.05 for Alg vs. Alg/PAAm, Alg, & Fib/PAAm. + P < 0.05 for Fib vs. PAAm, Alg/PAAm and Fib/PAAm.
Figure 2
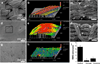
(A, B) SEM and 3D optical profile of 3D woven PCL scaffold surface. Outlined area in panel A denotes total scanned area in panel B. (C) Cross-sectional image of 3D woven PCL scaffold. (D, E) SEM and 3D optical profile of 3D woven PCL-Alg/PAAm IPN composite surface. Outlined area in panel D denotes total scanned area in panel E. (F) Cross-sectional image of 3D woven PCL-Alg/PAAm IPN composite scaffold. (G, H) SEM and 3D optical profile of Alg/PAAm IPN surface. (I) Average RMS of the 3D woven PCL scaffold (scaffold), Alg/PAAm IPN (hydrogel), and 3D woven PCL-Alg/PAAm IPN composite (composite) constructs. * P < 0.05 for hydrogel vs. scaffold & composite. + P < 0.05 for composite vs. scaffold and hydrogel. All scale bars = 200 µm.
Figure 3
(A) Young’s modulus of the PCL scaffold alone and the IPN hydrogel composites (All groups in this plot contain the PCL scaffold – the hydrogel was infused in the PCL scaffold to create the composite). * P < 0.05 for Alg/PAAm vs. all groups except Fib/PAAm. + P < 0.05 for Fib/PAAm vs. all groups except Alg/PAAm. (B) Aggregate modulus of IPN and single network hydrogels and their combination with the PCL scaffold to form composites. * P < 0.05 for Alg/PAAm composite vs. all groups. + P < 0.05 for Fib/AAM hydrogel vs. all groups. ** P < 0.05 for Fib/PAAm composite vs. Alg and Fib composites and scaffold alone. (C) Stress-strain behavior and (D) dynamic moduli of porcine articular cartilage, Alg/PAAm hydrogel, Alg/PAAm composite and woven scaffold.
Figure 4
The equilibrium coefficient of friction of different constructs tested against native articular cartilage. 3D woven PCL scaffold showed a relatively high coefficient of friction, whereas the Alg/PAAm IPN composite and Alg/PAAm hydrogel showed significantly lower coefficients of friction. * P < 0.05 for 3D woven PCL scaffold vs. hydrogel & composite.
Similar articles
- Composite Cellularized Structures Created from an Interpenetrating Polymer Network Hydrogel Reinforced by a 3D Woven Scaffold.
Moffat KL, Goon K, Moutos FT, Estes BT, Oswald SJ, Zhao X, Guilak F. Moffat KL, et al. Macromol Biosci. 2018 Oct;18(10):e1800140. doi: 10.1002/mabi.201800140. Epub 2018 Jul 24. Macromol Biosci. 2018. PMID: 30040175 Free PMC article. - Functional properties of cell-seeded three-dimensionally woven poly(epsilon-caprolactone) scaffolds for cartilage tissue engineering.
Moutos FT, Guilak F. Moutos FT, et al. Tissue Eng Part A. 2010 Apr;16(4):1291-301. doi: 10.1089/ten.TEA.2009.0480. Tissue Eng Part A. 2010. PMID: 19903085 Free PMC article. - Laponite nanoparticle-associated silated hydroxypropylmethyl cellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering.
Boyer C, Figueiredo L, Pace R, Lesoeur J, Rouillon T, Visage CL, Tassin JF, Weiss P, Guicheux J, Rethore G. Boyer C, et al. Acta Biomater. 2018 Jan;65:112-122. doi: 10.1016/j.actbio.2017.11.027. Epub 2017 Nov 8. Acta Biomater. 2018. PMID: 29128532 - Hydrogels as a Replacement Material for Damaged Articular Hyaline Cartilage.
Beddoes CM, Whitehouse MR, Briscoe WH, Su B. Beddoes CM, et al. Materials (Basel). 2016 Jun 3;9(6):443. doi: 10.3390/ma9060443. Materials (Basel). 2016. PMID: 28773566 Free PMC article. Review. - Hydrogels for the repair of articular cartilage defects.
Spiller KL, Maher SA, Lowman AM. Spiller KL, et al. Tissue Eng Part B Rev. 2011 Aug;17(4):281-99. doi: 10.1089/ten.TEB.2011.0077. Epub 2011 Jun 30. Tissue Eng Part B Rev. 2011. PMID: 21510824 Free PMC article. Review.
Cited by
- Engineering a highly elastic bioadhesive for sealing soft and dynamic tissues.
Ghovvati M, Baghdasarian S, Baidya A, Dhal J, Annabi N. Ghovvati M, et al. J Biomed Mater Res B Appl Biomater. 2022 Jul;110(7):1511-1522. doi: 10.1002/jbm.b.35012. Epub 2022 Feb 11. J Biomed Mater Res B Appl Biomater. 2022. PMID: 35148016 Free PMC article. - Functional tissue engineering of articular cartilage for biological joint resurfacing-The 2021 Elizabeth Winston Lanier Kappa Delta Award.
Guilak F, Estes BT, Moutos FT. Guilak F, et al. J Orthop Res. 2022 Aug;40(8):1721-1734. doi: 10.1002/jor.25223. Epub 2021 Dec 6. J Orthop Res. 2022. PMID: 34812518 Free PMC article. - Fiber reinforced GelMA hydrogel to induce the regeneration of corneal stroma.
Kong B, Chen Y, Liu R, Liu X, Liu C, Shao Z, Xiong L, Liu X, Sun W, Mi S. Kong B, et al. Nat Commun. 2020 Mar 18;11(1):1435. doi: 10.1038/s41467-020-14887-9. Nat Commun. 2020. PMID: 32188843 Free PMC article. - Bi-layered micro-fibre reinforced hydrogels for articular cartilage regeneration.
Castilho M, Mouser V, Chen M, Malda J, Ito K. Castilho M, et al. Acta Biomater. 2019 Sep 1;95:297-306. doi: 10.1016/j.actbio.2019.06.030. Epub 2019 Jun 22. Acta Biomater. 2019. PMID: 31233890 Free PMC article. - Advancements in textile techniques for cardiovascular tissue replacement and repair.
Bakare A, Mohanadas HP, Tucker N, Ahmed W, Manikandan A, Faudzi AAM, Mohamaddan S, Jaganathan SK. Bakare A, et al. APL Bioeng. 2024 Oct 17;8(4):041503. doi: 10.1063/5.0231856. eCollection 2024 Dec. APL Bioeng. 2024. PMID: 39431050 Free PMC article. Review.
References
- Mow VC, Ratcliffe A, Poole AR. Biomaterials. 1992;13:67. - PubMed
- Guilak F, Setton LA, Kraus VB. In: Principles and Practice of Orthopaedic Sports Medicine. Garrett WE, Speer K, Kirkendall D, editors. Philadelphia: Lippincott Williams and Wilkins; 2000. p. 53.
- Wang HQ, Ateshian GA. Journal of Biomechanics. 1997;30:771. - PubMed
- Soltz MA, Ateshian GA. Annals of biomedical engineering. 2000;28:150. - PubMed
Grants and funding
- R01 AR048852/AR/NIAMS NIH HHS/United States
- R01 AR048182/AR/NIAMS NIH HHS/United States
- P01 AR050245/AR/NIAMS NIH HHS/United States
- R01 AG015768/AG/NIA NIH HHS/United States
- R42 AR055042/AR/NIAMS NIH HHS/United States
- R41 AR055042/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources