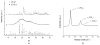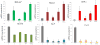Simultaneous bactericidal and osteogenic effect of nanoparticulate calcium phosphate powders loaded with clindamycin on osteoblasts infected with Staphylococcus aureus - PubMed (original) (raw)
Simultaneous bactericidal and osteogenic effect of nanoparticulate calcium phosphate powders loaded with clindamycin on osteoblasts infected with Staphylococcus aureus
Vuk Uskoković et al. Mater Sci Eng C Mater Biol Appl. 2014.
Abstract
Staphylococcus aureus internalized by bone cells and shielded from the immune system provides a reservoir of bacteria in recurring osteomyelitis. Its targeting by the antibiotic therapy may thus be more relevant for treating chronic bone infection than eliminating only the pathogens colonizing the bone matrix. Assessed was the combined osteogenic and antibacterial effect of clindamycin-loaded calcium phosphate nanoparticles of different monophasic compositions on co-cultures comprising osteoblasts infected with S. aureus. Antibiotic-carrying particles were internalized by osteoblasts and minimized the concentration of intracellular bacteria. In vitro treatments of the infected cells, however, could not prevent cell necrosis due to the formation of toxic byproducts of the degradation of the bacterium. Antibiotic-loaded particles had a positive morphological effect on osteoblasts per se, without reducing their viability, alongside stimulating the upregulation of expression of different bone growth markers in infected osteoblasts to a higher degree than achieved during the treatment with antibiotic only.
Keywords: Calcium phosphate; Controlled drug delivery; Nanoparticles; Osteogenesis; Osteomyelitis; Staphylococcus aureus.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
Fig. 1
Scanning electron micrographs of spherical and monodisperse HAP nanoparticles (a), forming compact microscopic blocks and capturing the adsorbed drug upon desiccation (b). (c) A transmission electron micrograph of a single nanoscopic particle of HAP with denoted defect-laden crystalline particle core and the largely amorphous, disorder surface atomic layers thereof. DCPA (d) and ACP (e) powders display similar nanoparticle size and shape characteristics.
Fig. 2
(a) XRD patterns of three different monophasic CAP nanopowders with different solubility products (Ksp) utilized in this study: (a) DCPA (pKsp = 7); (b) ACP (pKsp = 20 – 25); (c) HAP (pKsp = 114). (b) Raman spectrum of HAP in 900 – 1200 cm−1 region, showing three bands originating from the symmetrical (ν1) and asymmetrical (ν3) stretches of the phosphate ion and from the symmetrical stretch of the carbonate ion, at 961, 1048 and 1075 cm−1, respectively.
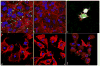
Fig. 3
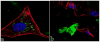
Z-stacked confocal optical images of fluorescently stained osteoblastic cell nuclei (blue) and cytoskeletal f-actin (red), invaded with S aureus (green): (a) positive control stained immediately following the 2 h infection, showing intracellular presence of the bacterium; (b) negative control at 48 h of incubation time; (c) positive control at 48 h of incubation time; co-cultured samples incubated with 2 mg/cm2 of HAP/CL (d), ACP/CL (e), and DCPA/CL (f) at 48 h of incubation time. The sizes of images are 270 × 270 μm (a, b), 450 × 450 μm (c, d, f), and 350 × 350 μm (e).
Fig. 4
(a) Bacterial colonies forming on sheep blood agar plates following streaking of lysates of cells invaded by S aureus and incubated with either no particles (control), or with CL-loaded HAP (HAP), or pure CL (CL); (b) the number of bacterial colonies counted following overnight incubation of streaked lysates of cells invaded by S aureus and incubated for different times (1, 8, 24 h, and 5 days) with either no particles (C+), or with CL-loaded HAP (HAP), CL-loaded ACP (ACP), CL-loaded DCPA (DCPA), or pure CL (CL); (c) optical densities at λ = 600 nm for BHI broths incubated for 4 h with fresh cell lysates obtained after a 4 h incubation with different particles; (d) average pixel intensity, directly relatable to the bacterial number in co-cultured samples, measured at the excitation wavelength of 488 nm and the detection wavelength range of 520 – 550 nm. Data are shown as arithmetic means with error bars representing standard deviation (* => p < 0.05 with respect to the C+ control group).
Fig. 4
(a) Bacterial colonies forming on sheep blood agar plates following streaking of lysates of cells invaded by S aureus and incubated with either no particles (control), or with CL-loaded HAP (HAP), or pure CL (CL); (b) the number of bacterial colonies counted following overnight incubation of streaked lysates of cells invaded by S aureus and incubated for different times (1, 8, 24 h, and 5 days) with either no particles (C+), or with CL-loaded HAP (HAP), CL-loaded ACP (ACP), CL-loaded DCPA (DCPA), or pure CL (CL); (c) optical densities at λ = 600 nm for BHI broths incubated for 4 h with fresh cell lysates obtained after a 4 h incubation with different particles; (d) average pixel intensity, directly relatable to the bacterial number in co-cultured samples, measured at the excitation wavelength of 488 nm and the detection wavelength range of 520 – 550 nm. Data are shown as arithmetic means with error bars representing standard deviation (* => p < 0.05 with respect to the C+ control group).
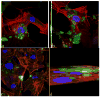
Fig. 5
Z-stacked confocal optical images of fluorescently stained osteoblastic cell nuclei (blue) and cytoskeletal f-actin (red), invaded with S aureus (green) previously made potentially resistant to CL therapy: (a) positive control stained immediately following the infection, showing intracellular presence of the bacterium; (b) negative control at 48 h of incubation time; (c) positive control at 48 h of incubation time; co-cultured samples incubated with 2 mg/cm2 of HAP/CL (d), ACP/CL (e), and DCPA/CL (f). The size of each image is 450 × 450 μm.
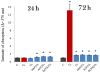
Fig. 6
Mitochondrial activity indicative of cell viability after different treatment intervals (24 and 72 h), normalized to the negative control (C−) and determined by the MTT assay for different types of CL-containing particles (HAP, ACP, DCPA), including CL alone and positive control, infected but not subjected to the treatment with the CL-containing particles (C+). Data normalized to the optical density at λ = 570 nm of the negative control are shown as arithmetic means with error bars representing standard deviation (* => p < 0.05 with respect to the C− control group).
Fig. 7
Single plane confocal optical images of fluorescently stained osteoblastic cell nucleus (blue) and cytoskeletal f-actin (red), and CL-containing CAP particles (green) of different monophasic compositions – HAP (a, d), ACP (b), and DCPA (c) – following 48 h (a) and 4 h (d) of incubation. The sizes of images are 270 × 270 μm.
Fig. 8
Single-plane confocal optical images of fluorescently stained HAP/CL, ACP/CL and DCPA/CL particles (green) and osteoblastic MC3T3-E1 cells (f-actin - red; nucleus - blue) following 21 days of incubation in the differentiation medium. The sizes of the images are 675 × 675 μm.
Fig. 9
Confocal optical micrographs of osteoblastic cells exhibiting disrupted cytoskeletal f-actin pattern (red) following incubation with Co-doped HAP particles (green) (a) as well as becoming morphologically deformed in contact with them (b). The sizes of the images are 270 × 270 μm.
Fig. 10
The comparative effect of pure HAP, pure CL and HAP loaded with CL (HAP/CL) on the mRNA expression of the housekeeping gene ACTB and different osteogenic markers - BGLAP, Runx2, BSP-1, ALP and Col I - in osteoblastic MC3T3-E1 cells infected with S aureus for 2 h and then incubated for 24 h with HAP, CL or HAP/CL. mRNA expression was detected by quantitative RT-polymerase chain reaction relative to the housekeeping gene ACTB. Data normalized to the expression of ACTB are shown as arithmetic means with error bars representing standard deviation. Genes significantly (p < 0.05) upregulated with respect to the control group are marked with *. Genes significantly (p < 0.05) downregulated with respect to the negative control group (C−) are marked with +.
Similar articles
- Effect of calcium phosphate particle shape and size on their antibacterial and osteogenic activity in the delivery of antibiotics in vitro.
Uskoković V, Batarni SS, Schweicher J, King A, Desai TA. Uskoković V, et al. ACS Appl Mater Interfaces. 2013 Apr 10;5(7):2422-31. doi: 10.1021/am4000694. Epub 2013 Mar 27. ACS Appl Mater Interfaces. 2013. PMID: 23484624 Free PMC article. - Osteogenic and antimicrobial nanoparticulate calcium phosphate and poly-(D,L-lactide-co-glycolide) powders for the treatment of osteomyelitis.
Uskoković V, Hoover C, Vukomanović M, Uskoković DP, Desai TA. Uskoković V, et al. Mater Sci Eng C Mater Biol Appl. 2013 Aug 1;33(6):3362-73. doi: 10.1016/j.msec.2013.04.023. Epub 2013 Apr 13. Mater Sci Eng C Mater Biol Appl. 2013. PMID: 23706222 Free PMC article. - Phase composition control of calcium phosphate nanoparticles for tunable drug delivery kinetics and treatment of osteomyelitis. II. Antibacterial and osteoblastic response.
Uskoković V, Desai TA. Uskoković V, et al. J Biomed Mater Res A. 2013 May;101(5):1427-36. doi: 10.1002/jbm.a.34437. Epub 2012 Oct 31. J Biomed Mater Res A. 2013. PMID: 23115128 Free PMC article. - Staphylococcus aureus vs. Osteoblast: Relationship and Consequences in Osteomyelitis.
Josse J, Velard F, Gangloff SC. Josse J, et al. Front Cell Infect Microbiol. 2015 Nov 26;5:85. doi: 10.3389/fcimb.2015.00085. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 26636047 Free PMC article. Review. - The Process of Osteoblastic Infection by Staphylococcus Aureus.
Wen Q, Gu F, Sui Z, Su Z, Yu T. Wen Q, et al. Int J Med Sci. 2020 May 29;17(10):1327-1332. doi: 10.7150/ijms.45960. eCollection 2020. Int J Med Sci. 2020. PMID: 32624688 Free PMC article. Review.
Cited by
- Empirical and theoretical insights into the structural effects of selenite doping in hydroxyapatite and the ensuing inhibition of osteoclasts.
Wu VM, Ahmed MK, Mostafa MS, Uskoković V. Wu VM, et al. Mater Sci Eng C Mater Biol Appl. 2020 Dec;117:111257. doi: 10.1016/j.msec.2020.111257. Epub 2020 Jul 6. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32919627 Free PMC article. - Bacteria-Targeted Clindamycin Loaded Polymeric Nanoparticles: Effect of Surface Charge on Nanoparticle Adhesion to MRSA, Antibacterial Activity, and Wound Healing.
Hasan N, Cao J, Lee J, Hlaing SP, Oshi MA, Naeem M, Ki MH, Lee BL, Jung Y, Yoo JW. Hasan N, et al. Pharmaceutics. 2019 May 15;11(5):236. doi: 10.3390/pharmaceutics11050236. Pharmaceutics. 2019. PMID: 31096709 Free PMC article. - Nanostructured platforms for the sustained and local delivery of antibiotics in the treatment of osteomyelitis.
Uskokovic V. Uskokovic V. Crit Rev Ther Drug Carrier Syst. 2015;32(1):1-59. doi: 10.1615/critrevtherdrugcarriersyst.2014010920. Crit Rev Ther Drug Carrier Syst. 2015. PMID: 25746204 Free PMC article. Review. - Bisphosphonate-Functionalized Hydroxyapatite Nanoparticles for the Delivery of the Bromodomain Inhibitor JQ1 in the Treatment of Osteosarcoma.
Wu VM, Mickens J, Uskoković V. Wu VM, et al. ACS Appl Mater Interfaces. 2017 Aug 9;9(31):25887-25904. doi: 10.1021/acsami.7b08108. Epub 2017 Jul 28. ACS Appl Mater Interfaces. 2017. PMID: 28731328 Free PMC article. - Meta-Analysis of Drug Delivery Approaches for Treating Intracellular Infections.
Shin S, Kwon S, Yeo Y. Shin S, et al. Pharm Res. 2022 Jun;39(6):1085-1114. doi: 10.1007/s11095-022-03188-z. Epub 2022 Feb 10. Pharm Res. 2022. PMID: 35146592 Free PMC article.
References
- Bisland SK, Chien C, Wilson BC, Burch S. Photochem Photobio Sci. 2006;5:31–38. - PubMed
- Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Office of the Surgeon General; Rockville, MD: 2004. - PubMed
- Paluska SA. Clin Fam Practice. 2004;6:127–156.
- King RW, Johnson DL. Stearns DA, Talavera F, Weiss EL, Halamka JD, Kulkarni R, editors. Osteomyelitis: eMedicine Medscape Reference. 2011 retrieved from http://emedicine.medscape.com/article/785020-overview#a0101.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical