Brain-gut microbiome interactions and functional bowel disorders - PubMed (original) (raw)
Review
Brain-gut microbiome interactions and functional bowel disorders
Emeran A Mayer et al. Gastroenterology. 2014 May.
Abstract
Alterations in the bidirectional interactions between the intestine and the nervous system have important roles in the pathogenesis of irritable bowel syndrome (IBS). A body of largely preclinical evidence suggests that the gut microbiota can modulate these interactions. A small and poorly defined role for dysbiosis in the development of IBS symptoms has been established through characterization of altered intestinal microbiota in IBS patients and reported improvement of subjective symptoms after its manipulation with prebiotics, probiotics, or antibiotics. It remains to be determined whether IBS symptoms are caused by alterations in brain signaling from the intestine to the microbiota or primary disruption of the microbiota, and whether they are involved in altered interactions between the brain and intestine during development. We review the potential mechanisms involved in the pathogenesis of IBS in different groups of patients. Studies are needed to better characterize alterations to the intestinal microbiome in large cohorts of well-phenotyped patients, and to correlate intestinal metabolites with specific abnormalities in gut-brain interactions.
Keywords: Dysbiosis; Irritable Bowel Syndrome; Probiotics.
Copyright © 2014 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures
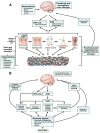
Figure 1. Bidirectional brain gut microbial interactions
A. Key components of brain gut microbial axis. A network of specialized target/transducer cells in the gut wall functions as an interface between the organism and the gut lumen. In response to external and bodily demands, the brain modulates individual cells (ECC – enterochromaffin cells; SMC – smooth muscle cells; ICC – interstitial cells of Cajal) within this network via the branches of the autonomic nervous system (ANS) (sympathetic and parasympathetic/vagal efferents) and the hypothalamic pituitary adrenal (HPA) axis. Such modulation can be transient (e.g. in response to transient perturbations) or longlasting (in response to chronically altered brain output). The microbiota are in constant bidirectional communication with this interface via multiple signaling pathways, and this communication is modulated in response to perturbations of the microbiota, or the brain. The integrated output of the brain gut microbial interface is transmitted to the brain via multiple afferent signaling pathways, including endocrine and neurocrine (vagal, spinal afferents) pathways. While acute alterations in this interoceptive feedback result in transient functional brain changes, chronic alterations are associated with neuroplastic brain changes. B. Functional and symptom-related consequences of brain gut microbial interactions. Several intestinal processes with possible relevance for IBS symptoms can be modulated both the brain (via the ANS, including its enteric nervous system [ENS] branch) and by signals from the microbiota. Microbiota generated molecules can signal to the brain indicrectly via activation of vagal (and possibly spinal) afferent nerve pathways, by microbiota stimulated cytokine and neurotransmitter release from immune or enteroendocrine cells, or such signals may reach the brain via an endocrine route. Microbiota gut brain signaling may contribute to the generation of abdominal pain and discomfort, while microbiota mediated modulation of enteric reflexes is likely to play a role in the pathophysiology of altered bowel habits.
Similar articles
- Microbiota-host interactions in irritable bowel syndrome: epithelial barrier, immune regulation and brain-gut interactions.
Hyland NP, Quigley EM, Brint E. Hyland NP, et al. World J Gastroenterol. 2014 Jul 21;20(27):8859-66. doi: 10.3748/wjg.v20.i27.8859. World J Gastroenterol. 2014. PMID: 25083059 Free PMC article. Review. - Unraveling the ties between irritable bowel syndrome and intestinal microbiota.
Hong SN, Rhee PL. Hong SN, et al. World J Gastroenterol. 2014 Mar 14;20(10):2470-81. doi: 10.3748/wjg.v20.i10.2470. World J Gastroenterol. 2014. PMID: 24627584 Free PMC article. Review. - Intestinal microbiota in pathophysiology and management of irritable bowel syndrome.
Lee KN, Lee OY. Lee KN, et al. World J Gastroenterol. 2014 Jul 21;20(27):8886-97. doi: 10.3748/wjg.v20.i27.8886. World J Gastroenterol. 2014. PMID: 25083061 Free PMC article. Review. - Is irritable bowel syndrome an infectious disease?
Thompson JR. Thompson JR. World J Gastroenterol. 2016 Jan 28;22(4):1331-4. doi: 10.3748/wjg.v22.i4.1331. World J Gastroenterol. 2016. PMID: 26819502 Free PMC article. Review. - Pathological and therapeutic interactions between bacteriophages, microbes and the host in inflammatory bowel disease.
Babickova J, Gardlik R. Babickova J, et al. World J Gastroenterol. 2015 Oct 28;21(40):11321-30. doi: 10.3748/wjg.v21.i40.11321. World J Gastroenterol. 2015. PMID: 26525290 Free PMC article. Review.
Cited by
- Intestinal Microbiome in Irritable Bowel Syndrome before and after Gut-Directed Hypnotherapy.
Peter J, Fournier C, Keip B, Rittershaus N, Stephanou-Rieser N, Durdevic M, Dejaco C, Michalski M, Moser G. Peter J, et al. Int J Mol Sci. 2018 Nov 16;19(11):3619. doi: 10.3390/ijms19113619. Int J Mol Sci. 2018. PMID: 30453528 Free PMC article. - Analysis of the gut microbiota composition of myostatin mutant cattle prepared using CRISPR/Cas9.
Wen T, Mao C, Gao L. Wen T, et al. PLoS One. 2022 Mar 4;17(3):e0264849. doi: 10.1371/journal.pone.0264849. eCollection 2022. PLoS One. 2022. PMID: 35245313 Free PMC article. - Development of the Pediatric Gut Microbiome: Impact on Health and Disease.
Ihekweazu FD, Versalovic J. Ihekweazu FD, et al. Am J Med Sci. 2018 Nov;356(5):413-423. doi: 10.1016/j.amjms.2018.08.005. Epub 2018 Aug 21. Am J Med Sci. 2018. PMID: 30384950 Free PMC article. Review. - Spinal anesthesia alleviates dextran sodium sulfate-induced colitis by modulating the gut microbiota.
Hong Y, Zhao J, Chen YR, Huang ZH, Hou LD, Shen B, Xin Y. Hong Y, et al. World J Gastroenterol. 2022 Mar 28;28(12):1239-1256. doi: 10.3748/wjg.v28.i12.1239. World J Gastroenterol. 2022. PMID: 35431512 Free PMC article. - Rationale of Probiotic Supplementation during Pregnancy and Neonatal Period.
Baldassarre ME, Palladino V, Amoruso A, Pindinelli S, Mastromarino P, Fanelli M, Di Mauro A, Laforgia N. Baldassarre ME, et al. Nutrients. 2018 Nov 6;10(11):1693. doi: 10.3390/nu10111693. Nutrients. 2018. PMID: 30404227 Free PMC article. Review.
References
- Frazier TH, DiBaise JK, McClain CJ. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. JPEN J Parenter Enteral Nutr. 2011;35:14S–20S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI100914/AI/NIAID NIH HHS/United States
- R01 AI100914/AI/NIAID NIH HHS/United States
- R01 DK048351/DK/NIDDK NIH HHS/United States
- P30 DK056338/DK/NIDDK NIH HHS/United States
- DK56338/DK/NIDDK NIH HHS/United States
- DK 64531/DK/NIDDK NIH HHS/United States
- DK 48351/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources