Progression of brain atrophy in spinocerebellar ataxia type 2: a longitudinal tensor-based morphometry study - PubMed (original) (raw)
. 2014 Feb 25;9(2):e89410.
doi: 10.1371/journal.pone.0089410. eCollection 2014.
Stefano Diciotti [ 2](#full-view-affiliation-2 ""Mario Serio" Department of Experimental and Clinical Biomedical Sciences, University of Florence, Florence, Italy ; Department of Electrical, Electronic, and Information Engineering "Guglielmo Marconi", University of Bologna, Cesena, Italy."), Marco Giannelli [ 3](#full-view-affiliation-3 "Unit of Medical Physics, Pisa University Hospital "Azienda Ospedaliero-Universitaria Pisana", Pisa, Italy."), Andrea Ginestroni 4, Andrea Soricelli 5, Emanuele Nicolai 6, Marco Aiello 6, Carlo Tessa 7, Lucia Galli 8, Maria Teresa Dotti 9, Silvia Piacentini 10, Elena Salvatore 11, Nicola Toschi [ 12](#full-view-affiliation-12 "Medical Physics Section, Department of Biomedicine and Prevention, University of Rome "Tor Vergata", Rome, Italy ; Department of Radiology, Athinoula A. Martinos Center for Biomedical Imaging, Boston, Massachusetts, United States of America ; Harvard Medical School, Boston, Massachusetts, United States of America.")
Affiliations
- PMID: 24586758
- PMCID: PMC3934889
- DOI: 10.1371/journal.pone.0089410
Progression of brain atrophy in spinocerebellar ataxia type 2: a longitudinal tensor-based morphometry study
Mario Mascalchi et al. PLoS One. 2014.
Abstract
Spinocerebellar ataxia type 2 (SCA2) is the second most frequent autosomal dominant inherited ataxia worldwide. We investigated the capability of magnetic resonance imaging (MRI) to track in vivo progression of brain atrophy in SCA2 by examining twice 10 SCA2 patients (mean interval 3.6 years) and 16 age- and gender-matched healthy controls (mean interval 3.3 years) on the same 1.5 T MRI scanner. We used T1-weighted images and tensor-based morphometry (TBM) to investigate volume changes and the Inherited Ataxia Clinical Rating Scale to assess the clinical deficit. With respect to controls, SCA2 patients showed significant higher atrophy rates in the midbrain, including substantia nigra, basis pontis, middle cerebellar peduncles and posterior medulla corresponding to the gracilis and cuneatus tracts and nuclei, cerebellar white matter (WM) and cortical gray matter (GM) in the inferior portions of the cerebellar hemisphers. No differences in WM or GM volume loss were observed in the supratentorial compartment. TBM findings did not correlate with modifications of the neurological deficit. In conclusion, MRI volumetry using TBM is capable of demonstrating the progression of pontocerebellar atrophy in SCA2, supporting a possible role of MRI as biomarker in future trials.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
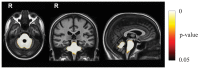
Figure 1. Results of the baseline between group (SCA2 vs. controls) TBM analysis.
Voxel-wise corrected p-value maps (threshold-free cluster enhancement, TFCE), testing the null hypothesis of zero differences in |J| baseline between SCA2 patients and healthy controls. Highlighted clusters indicate significantly (p<0.05) more pronounced mean atrophy in SCA2 patients when compared to healthy controls (i.e. |J| baseline in SCA2 patients significantly lower than |J| baseline in healthy controls). All maps are overlayed on population-specific T1 template. These maps show significant symmetric atrophic changes in SCA2 patients (with respect to controls) in the brainstem, middle cerebellar peduncels, and cerebellar WM and adjacent cortical GM. No significant differences are observed in the supratentorial compartment.
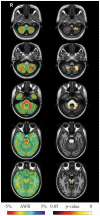
Figure 2. Results of longitudinal between group (SCA2 vs. controls) TBM analysis.
Left pane: Sample axial views of the difference in average longitudinal warp rate (ΔWR) maps between SCA2 patients and healthy controls, where red indicates local atrophy and blue indicates local enlargement. Right pane: Voxel-wise corrected p-value maps (threshold-free cluster enhancement, TFCE), testing the null hypothesis of zero differences in WR between SCA2 patients and healthy controls. Highlighted clusters indicate significantly (p<0.05) more pronounced mean atrophy in SCA2 patients when compared to healthy controls (i.e. WR in SCA2 patients significantly lower than WR in control subjects). All maps are overlayed on the population-specific T1 template. SCA2 patients exhibit significant volume loss (higher atrophy rates with respect to controls) in the midbrain (substantia nigra and medial lemniscus, bilaterally, right lateral lemniscus and central region corresponding to decussation of the superior cerebellar peduncles), the entire basis pontis, the middle cerebellar peduncles and posterior medulla corresponding to the in the gracilis and cuneatus tracts and nuclei. The cerebellum shows loss of WM in the hemispheric and peridentate region and of GM in the cerebellar cortex of the inferior portions of the cerebellar hemisphers.
Similar articles
- Structural Complexity of the Cerebellum and Cerebral Cortex is Reduced in Spinocerebellar Ataxia Type 2.
Marzi C, Ciulli S, Giannelli M, Ginestroni A, Tessa C, Mascalchi M, Diciotti S. Marzi C, et al. J Neuroimaging. 2018 Nov;28(6):688-693. doi: 10.1111/jon.12534. Epub 2018 Jul 5. J Neuroimaging. 2018. PMID: 29975004 - Brain structural damage in spinocerebellar ataxia type 2. A voxel-based morphometry study.
Della Nave R, Ginestroni A, Tessa C, Cosottini M, Giannelli M, Salvatore E, Sartucci F, De Michele G, Dotti MT, Piacentini S, Mascalchi M. Della Nave R, et al. Mov Disord. 2008 Apr 30;23(6):899-903. doi: 10.1002/mds.21982. Mov Disord. 2008. PMID: 18311829 - Brain white matter damage in SCA1 and SCA2. An in vivo study using voxel-based morphometry, histogram analysis of mean diffusivity and tract-based spatial statistics.
Della Nave R, Ginestroni A, Tessa C, Salvatore E, De Grandis D, Plasmati R, Salvi F, De Michele G, Dotti MT, Piacentini S, Mascalchi M. Della Nave R, et al. Neuroimage. 2008 Oct 15;43(1):10-9. doi: 10.1016/j.neuroimage.2008.06.036. Epub 2008 Jul 11. Neuroimage. 2008. PMID: 18672073 - Neuroimaging Applications in Chronic Ataxias.
Mascalchi M, Vella A. Mascalchi M, et al. Int Rev Neurobiol. 2018;143:109-162. doi: 10.1016/bs.irn.2018.09.011. Epub 2018 Oct 29. Int Rev Neurobiol. 2018. PMID: 30473193 Review. - Progressive cerebellar atrophy: hereditary ataxias and disorders with spinocerebellar degeneration.
Wolf NI, Koenig M. Wolf NI, et al. Handb Clin Neurol. 2013;113:1869-78. doi: 10.1016/B978-0-444-59565-2.00057-5. Handb Clin Neurol. 2013. PMID: 23622410 Review.
Cited by
- Viewpoint: spinocerebellar ataxias as diseases of Purkinje cell dysfunction rather than Purkinje cell loss.
Kapfhammer JP, Shimobayashi E. Kapfhammer JP, et al. Front Mol Neurosci. 2023 Jun 22;16:1182431. doi: 10.3389/fnmol.2023.1182431. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37426070 Free PMC article. Review. - Brain atrophy measures in preclinical and manifest spinocerebellar ataxia type 2.
Reetz K, Rodríguez-Labrada R, Dogan I, Mirzazade S, Romanzetti S, Schulz JB, Cruz-Rivas EM, Alvarez-Cuesta JA, Aguilera Rodríguez R, Gonzalez Zaldivar Y, Auburger G, Velázquez-Pérez L. Reetz K, et al. Ann Clin Transl Neurol. 2018 Jan 7;5(2):128-137. doi: 10.1002/acn3.504. eCollection 2018 Feb. Ann Clin Transl Neurol. 2018. PMID: 29468174 Free PMC article. - Degenerative Ataxias: challenges in clinical research.
Subramony SH. Subramony SH. Ann Clin Transl Neurol. 2016 Nov 17;4(1):53-60. doi: 10.1002/acn3.374. eCollection 2017 Jan. Ann Clin Transl Neurol. 2016. PMID: 28078315 Free PMC article. Review. - Tracking longitudinal thalamic volume changes during early stages of SCA1 and SCA2.
Grisoli M, Nigri A, Medina Carrion JP, Palermo S, Demichelis G, Giacosa C, Mongelli A, Fichera M, Nanetti L, Mariotti C. Grisoli M, et al. Radiol Med. 2024 Aug;129(8):1215-1223. doi: 10.1007/s11547-024-01839-2. Epub 2024 Jul 2. Radiol Med. 2024. PMID: 38954239 Free PMC article. - On the Cut-Off Value of the Anteroposterior Diameter of the Midbrain Atrophy in Spinocerebellar Ataxia Type 2 Patients.
Álvarez-Cuesta JA, Mora-Batista C, Reyes-Carreto R, Carrillo-Rodes FJ, Fitz SJT, González-Zaldivar Y, Vargas-De-León C. Álvarez-Cuesta JA, et al. Brain Sci. 2024 Jan 5;14(1):53. doi: 10.3390/brainsci14010053. Brain Sci. 2024. PMID: 38248268 Free PMC article.
References
- Auburger GW (2012) Spinocerebellar ataxia type 2. Handb Clin Neurol 103: 423–436. - PubMed
- Velazquez-Perez L, Rodriguez-Labrada R, Garcia-Rodriguez JC, Almaguer-Mederos LE, Cruz-Marino T, et al. (2011) A comprehensive review of spinocerebellar ataxia type 2 in Cuba. Cerebellum 10: 184–198. - PubMed
- Takahashi T, Katada S, Onodera O (2010) Polyglutamine diseases: where does toxicity come from? what is toxicity? where are we going? J Mol Cell Biol 2: 180–191. - PubMed
- Brenneis C, Bosch SM, Schocke M, Wenning GK, Poewe W (2003) Atrophy pattern in SCA2 determined by voxel-based morphometry. Neuroreport 14: 1799–1802. - PubMed
- Burk K, Abele M, Fetter M, Dichgans J, Skalej M, et al. (1996) Autosomal dominant cerebellar ataxia type I clinical features and MRI in families with SCA1, SCA2 and SCA3. Brain 119 (Pt 5): 1497–1505. - PubMed
MeSH terms
Grants and funding
The authors have no support or funding to report.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials