Ablation of the oncogenic transcription factor ERG by deubiquitinase inhibition in prostate cancer - PubMed (original) (raw)
. 2014 Mar 18;111(11):4251-6.
doi: 10.1073/pnas.1322198111. Epub 2014 Mar 3.
Rahul K Kollipara, Nishi Srivastava, Rui Li, Preethi Ravindranathan, Elizabeth Hernandez, Eva Freeman, Caroline G Humphries, Payal Kapur, Yair Lotan, Ladan Fazli, Martin E Gleave, Stephen R Plymate, Ganesh V Raj, Jer-Tsong Hsieh, Ralf Kittler
Affiliations
- PMID: 24591637
- PMCID: PMC3964108
- DOI: 10.1073/pnas.1322198111
Ablation of the oncogenic transcription factor ERG by deubiquitinase inhibition in prostate cancer
Shan Wang et al. Proc Natl Acad Sci U S A. 2014.
Abstract
The transcription factor E-twenty-six related gene (ERG), which is overexpressed through gene fusion with the androgen-responsive gene transmembrane protease, serine 2 (TMPRSS2) in ∼40% of prostate tumors, is a key driver of prostate carcinogenesis. Ablation of ERG would disrupt a key oncogenic transcriptional circuit and could be a promising therapeutic strategy for prostate cancer treatment. Here, we show that ubiquitin-specific peptidase 9, X-linked (USP9X), a deubiquitinase enzyme, binds ERG in VCaP prostate cancer cells expressing TMPRSS2-ERG and deubiquitinates ERG in vitro. USP9X knockdown resulted in increased levels of ubiquitinated ERG and was coupled with depletion of ERG. Treatment with the USP9X inhibitor WP1130 resulted in ERG degradation both in vivo and in vitro, impaired the expression of genes enriched in ERG and prostate cancer relevant gene signatures in microarray analyses, and inhibited growth of ERG-positive tumors in three mouse xenograft models. Thus, we identified USP9X as a potential therapeutic target in prostate cancer cells and established WP1130 as a lead compound for the development of ERG-depleting drugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
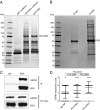
Fig. 1.
Identification of USP9X as an ERG-binding protein. (A and B) Detection of ERG-binding proteins by pulldown with recombinant GST-ERG (A) or immunoprecipitation with an antibody against ERG (B) from VCaP whole cell extract. Proteins were separated by SDS/PAGE and detected by Coomassie blue staining. The asterisk indicates IgG heavy chain. (C) Coimmunoprecipitation of USP9X with endogenous ERG in VCaP cells with an antibody directed against ERG. Immunoblotting was performed with antibodies against ERG and USP9X. (D) USP9X protein expression in benign prostate (n = 37), ERG-negative (n = 63), and ERG-positive (n = 34) prostate cancer specimens (P values, Student's t test).
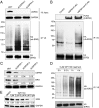
Fig. 2.
USP9X stabilizes ERG by deubiquitination, which is inhibited by WP1130. (A) USP9X knockdown increases ERG ubiquitination. HeLa cells were transfected with siRNAs against USP9X or control siRNA (siNT); after 72 h, these cells were cotransfected with ERG-V5 and HA-ubiquitin expression constructs. After 24 h, ERG-V5 was immunoprecipitated, and immunoblotting was performed with antibodies against V5 and HA to detect ubiquitinated ERG (ub-ERG), or with antibodies against USP9X and GAPDH for input to monitor the efficiency of the USP9X knockdown. The asterisk indicates monoubiquitinated ERG (65 kDa). (B) USP9X deubiquitinates ERG in vitro. Ubiquitinated ERG-V5 was incubated with wild-type USP9X-FLAG, mutant USP9X-mut-FLAG, or mock (control), followed by immunoblotting. (C) USP9X knockdown decreases ERG levels in VCaP cells. VCaP cells were transfected with siRNAs directed against USP9X or siNT. (D) WP1130 increases the levels of ubiquitinated ERG. HeLa cells expressing ERG-V5 and HA-ubiquitin were treated with 5 µM WP1130 for 0–4 h before ERG-V5 immunoprecipitation. (E) WP1130 causes ERG depletion in VCaP cells. VCaP cells were treated with WP1130 for 24 h. Quantification of triplicate immunoblot analysis for C and E is shown in
SI Appendix, Table S7
.
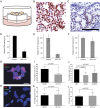
Fig. 3.
Effects of WP1130 treatment on prostate cancer cells. (A_–_F) Prostate tumor explants respond to WP1130 treatment. Prostate tumor ex vivo cultures are generated from freshly resected tumor specimens and grown on gelatin sponges in culture (A). ERG IHC staining in ERG+ prostate tumor explants following incubation for 24 h in either the presence of vehicle (DMSO) (B) or 10 µM WP1130 (C). (Scale bar: 100 µm.) Quantification of ERG levels in ERG+ tumors (n = 5) (D). WP1130 treatment strongly inhibits proliferation in ERG_+_ tumors (n = 5) (E) but not in ERG− tumors (n = 7) (F) as indicated by Ki-67 staining. Error bars represent SD. (G_–_L) WP1130 treatment reduces DNA damage. VCaP cells were treated for 48 h with DMSO (G) or 2 µM WP1130 (H) before immunofluorescence analysis of ERG, γH2AX, and DNA (DAPI). Quantification of γH2AX after WP1130/control treatment (I), RNAi knockdown of ERG and USP9X (J) in VCaP cells, or WP1130/control treatment in PC3 (K) and Du-145 (L) cells. Error bars represent SD (P values, Student's t test).
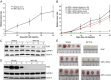
Fig. 4.
Effects of WP1130 on ERG levels and tumor growth in vivo. (A and B) WP1130 inhibits the growth of ERG-overexpressing tumors in mice. Mice xenografted with ERG-overexpressing VCaP cells (A) or 22Rv1-ERG and 22Rv1-vector cells (B) were treated upon appearance of tumors with 40 mg/kg WP1130 or vehicle (i.p.) every other day beginning on day 27 (A) or 12 (B) after cell injection. Error bars represent SEM. (C and D) WP1130 causes ERG depletion in vivo. Tumors were obtained from VCaP (C) and 22Rv1-ERG (D) tumor-bearing mice 24 h after injection with vehicle or WP1130 (40 mg/kg). Immunoblotting was performed with antibodies against ERG, GAPDH, and COX IV (human protein-specific antibody). (E) Appearance of xenograft VCaP, 22Rv1-ERG, and 22Rv1-vector tumors at the end of the treatment course with vehicle or WP1130 (40 mg/kg). Treatment with WP1130 was not associated with any apparent toxicity (e.g., weight loss or behavioral changes).
Similar articles
- The ubiquitin ligase TRIM25 targets ERG for degradation in prostate cancer.
Wang S, Kollipara RK, Humphries CG, Ma SH, Hutchinson R, Li R, Siddiqui J, Tomlins SA, Raj GV, Kittler R. Wang S, et al. Oncotarget. 2016 Oct 4;7(40):64921-64931. doi: 10.18632/oncotarget.11915. Oncotarget. 2016. PMID: 27626314 Free PMC article. - Genetic interaction between Tmprss2-ERG gene fusion and Nkx3.1-loss does not enhance prostate tumorigenesis in mouse models.
Linn DE, Bronson RT, Li Z. Linn DE, et al. PLoS One. 2015 Mar 17;10(3):e0120628. doi: 10.1371/journal.pone.0120628. eCollection 2015. PLoS One. 2015. PMID: 25780911 Free PMC article. - Synergistic Activity with NOTCH Inhibition and Androgen Ablation in ERG-Positive Prostate Cancer Cells.
Mohamed AA, Tan SH, Xavier CP, Katta S, Huang W, Ravindranath L, Jamal M, Li H, Srivastava M, Srivatsan ES, Sreenath TL, McLeod DG, Srinivasan A, Petrovics G, Dobi A, Srivastava S. Mohamed AA, et al. Mol Cancer Res. 2017 Oct;15(10):1308-1317. doi: 10.1158/1541-7786.MCR-17-0058. Epub 2017 Jun 12. Mol Cancer Res. 2017. PMID: 28607007 Free PMC article. - Context-dependent function of the deubiquitinating enzyme USP9X in pancreatic ductal adenocarcinoma.
Cox JL, Wilder PJ, Wuebben EL, Ouellette MM, Hollingsworth MA, Rizzino A. Cox JL, et al. Cancer Biol Ther. 2014 Aug;15(8):1042-52. doi: 10.4161/cbt.29182. Epub 2014 May 19. Cancer Biol Ther. 2014. PMID: 24841553 Free PMC article. - [The progress of TMPRSS2-ETS gene fusions and their mechanism in prostate cancer].
Guo XQ, Gui YT, Cai ZM. Guo XQ, et al. Yi Chuan. 2011 Feb;33(2):117-22. doi: 10.3724/sp.j.1005.2011.00117. Yi Chuan. 2011. PMID: 21377967 Review. Chinese.
Cited by
- Roles of deubiquitinases in urologic cancers (Review).
Wu L, Wang J, Chai L, Chen J, Jin X. Wu L, et al. Oncol Lett. 2024 Oct 14;28(6):609. doi: 10.3892/ol.2024.14743. eCollection 2024 Dec. Oncol Lett. 2024. PMID: 39525605 Free PMC article. Review. - The deubiquitinase USP9X regulates RIT1 protein abundance and oncogenic phenotypes.
Riley AK, Grant M, Snell A, Cromwell E, Vichas A, Moorthi S, Rominger C, Modukuri SP, Urisman A, Castel P, Wan L, Berger AH. Riley AK, et al. iScience. 2024 Jul 14;27(8):110499. doi: 10.1016/j.isci.2024.110499. eCollection 2024 Aug 16. iScience. 2024. PMID: 39161959 Free PMC article. - USP3 promotes osteosarcoma progression via deubiquitinating EPHA2 and activating the PI3K/AKT signaling pathway.
Li A, Wang S, Nie J, Xiao S, Xie X, Zhang Y, Tong W, Yao G, Liu N, Dan F, Shu Z, Liu J, Liu Z, Yang F. Li A, et al. Cell Death Dis. 2024 Mar 26;15(3):235. doi: 10.1038/s41419-024-06624-7. Cell Death Dis. 2024. PMID: 38531846 Free PMC article. - Regulation of post-translational modification of PD-L1 and advances in tumor immunotherapy.
Feng C, Zhang L, Chang X, Qin D, Zhang T. Feng C, et al. Front Immunol. 2023 Jul 24;14:1230135. doi: 10.3389/fimmu.2023.1230135. eCollection 2023. Front Immunol. 2023. PMID: 37554324 Free PMC article. Review. - Emerging role of ubiquitination/deubiquitination modification of PD-1/PD-L1 in cancer immunotherapy.
Ding P, Ma Z, Fan Y, Feng Y, Shao C, Pan M, Zhang Y, Huang D, Han J, Hu Y, Yan X. Ding P, et al. Genes Dis. 2022 Feb 18;10(3):848-863. doi: 10.1016/j.gendis.2022.01.002. eCollection 2023 May. Genes Dis. 2022. PMID: 37396527 Free PMC article. Review.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. - PubMed
- Tomlins SA, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310(5748):644–648. - PubMed
- Mehra R, et al. Comprehensive assessment of TMPRSS2 and ETS family gene aberrations in clinically localized prostate cancer. Mod Pathol. 2007;20(5):538–544. - PubMed
- Demichelis F, et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26(31):4596–4599. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical