How voltage-gated calcium channels gate forms of homeostatic synaptic plasticity - PubMed (original) (raw)
Review
How voltage-gated calcium channels gate forms of homeostatic synaptic plasticity
C Andrew Frank. Front Cell Neurosci. 2014.
Abstract
Throughout life, animals face a variety of challenges such as developmental growth, the presence of toxins, or changes in temperature. Neuronal circuits and synapses respond to challenges by executing an array of neuroplasticity paradigms. Some paradigms allow neurons to up- or downregulate activity outputs, while countervailing ones ensure that outputs remain within appropriate physiological ranges. A growing body of evidence suggests that homeostatic synaptic plasticity (HSP) is critical in the latter case. Voltage-gated calcium channels gate forms of HSP. Presynaptically, the aggregate data show that when synapse activity is weakened, homeostatic signaling systems can act to correct impairments, in part by increasing calcium influx through presynaptic CaV2-type channels. Increased calcium influx is often accompanied by parallel increases in the size of active zones and the size of the readily releasable pool of presynaptic vesicles. These changes coincide with homeostatic enhancements of neurotransmitter release. Postsynaptically, there is a great deal of evidence that reduced network activity and loss of calcium influx through CaV1-type calcium channels also results in adaptive homeostatic signaling. Some adaptations drive presynaptic enhancements of vesicle pool size and turnover rate via retrograde signaling, as well as de novo insertion of postsynaptic neurotransmitter receptors. Enhanced calcium influx through CaV1 after network activation or single cell stimulation can elicit the opposite response-homeostatic depression via removal of excitatory receptors. There exist intriguing links between HSP and calcium channelopathies-such as forms of epilepsy, migraine, ataxia, and myasthenia. The episodic nature of some of these disorders suggests alternating periods of stable and unstable function. Uncovering information about how calcium channels are regulated in the context of HSP could be relevant toward understanding these and other disorders.
Keywords: CaV1 channels; CaV2 channels; VGCCs; calcium channelopathies; homeostatic synaptic plasticity; neurotransmitter release; synaptic growth; synaptic scaling.
Figures
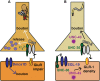
Figure 1
Mammalian CaV1 and CaV2 channels play central roles in forms of homeostatic plasticity. These two cartoons attempt to synthesize knowledge of mammalian pre- and postsynaptic homeostatic plasticity mechanisms involving CaV1 and CaV2 calcium channels in preparations like cultured hippocampal neurons. The cartoons are not intended to depict a single synaptic preparation or universally conserved mechanism, though some molecular responses may be widely conserved. (A) Homeostatic potentiation of synapse function. Inhibition of synaptic activity or postsynaptic CaV1 calcium influx results in multiple changes, including postsynaptic signaling through molecules like adenylate cyclase 1 (AC1) or guanylate kinase-associated protein (GKAP) to drive activating mechanisms, such as glutamate receptor insertion. Trans-synaptic signaling controlled by factors like Target of Rapamycin (TOR) and Brain-Derived Neurotrophic Factor (BDNF) can trigger enhanced presynaptic release probability. From a variety of systems there is evidence for enhanced presynaptic calcium influx through CaV2—which may require diminishment of cyclin-dependent kinase 5 (CDK5) function—as well as an enhanced readily releasable pool of presynaptic vesicles. (B) Homeostatic downscaling of synapse function. Synaptic activation (e.g., through GABA receptor blockade) and/or enhanced postsynaptic calcium influx through CaV1 results in the activation of diverse pathways, such as those mediated by calcium/calmodulin-dependent kinases (CaMK), as well as the Protein Phosphatase 1 (PP1) inhibitor, I-2. This can result in removal of excitatory glutamate receptors from the synapse. Presynaptically, there is evidence of diminished calcium influx through CaV2, and thus, diminished evoked presynaptic release.
Figure 2
Invertebrate models of CaV-directed homeostatic plasticity. Inspection of the Drosophila melanogaster NMJ has provided a wealth of information regarding the roles VGCCs and associated molecules play in homeostatic plasticity, as has examination of C. elegans preparations, such as the ventral nerve cord (VNC) or the NMJ. (A) Drosophila NMJ. Pharmacological or genetic impairment of postsynaptic glutamate receptors triggers a retrograde signaling process that results in enhanced presynaptic Cac/CaV2 function and increased neurotransmitter release. Additionally, Cac/CaV2, α2δ, and Dmca1D/CaV1 all affect synaptic bouton development or maturation at the NMJ. (B) C. elegans synapses. At the C. elegans VNC, the coordinated functions of UNC-2/CaV2, EGL-19/CaV1, UNC-36/α2δ and UNC-43/CaMKII ensure proper coupling of GLR-1 glutamate receptor density to developmental growth.
Similar articles
- Homeostatic plasticity at the Drosophila neuromuscular junction.
Frank CA. Frank CA. Neuropharmacology. 2014 Mar;78:63-74. doi: 10.1016/j.neuropharm.2013.06.015. Epub 2013 Jun 24. Neuropharmacology. 2014. PMID: 23806804 Free PMC article. Review. - Endogenous Tagging Reveals Differential Regulation of Ca2+ Channels at Single Active Zones during Presynaptic Homeostatic Potentiation and Depression.
Gratz SJ, Goel P, Bruckner JJ, Hernandez RX, Khateeb K, Macleod GT, Dickman D, O'Connor-Giles KM. Gratz SJ, et al. J Neurosci. 2019 Mar 27;39(13):2416-2429. doi: 10.1523/JNEUROSCI.3068-18.2019. Epub 2019 Jan 28. J Neurosci. 2019. PMID: 30692227 Free PMC article. - RIM controls homeostatic plasticity through modulation of the readily-releasable vesicle pool.
Müller M, Liu KS, Sigrist SJ, Davis GW. Müller M, et al. J Neurosci. 2012 Nov 21;32(47):16574-85. doi: 10.1523/JNEUROSCI.0981-12.2012. J Neurosci. 2012. PMID: 23175813 Free PMC article. - α-Neurexins Together with α2δ-1 Auxiliary Subunits Regulate Ca2+ Influx through Cav2.1 Channels.
Brockhaus J, Schreitmüller M, Repetto D, Klatt O, Reissner C, Elmslie K, Heine M, Missler M. Brockhaus J, et al. J Neurosci. 2018 Sep 19;38(38):8277-8294. doi: 10.1523/JNEUROSCI.0511-18.2018. Epub 2018 Aug 13. J Neurosci. 2018. PMID: 30104341 Free PMC article. - Calcium Channels, Synaptic Plasticity, and Neuropsychiatric Disease.
Nanou E, Catterall WA. Nanou E, et al. Neuron. 2018 May 2;98(3):466-481. doi: 10.1016/j.neuron.2018.03.017. Neuron. 2018. PMID: 29723500 Review.
Cited by
- Glutamatergic Mechanisms Associated with Seizures and Epilepsy.
Barker-Haliski M, White HS. Barker-Haliski M, et al. Cold Spring Harb Perspect Med. 2015 Jun 22;5(8):a022863. doi: 10.1101/cshperspect.a022863. Cold Spring Harb Perspect Med. 2015. PMID: 26101204 Free PMC article. Review. - Drosophila CaV2 channels harboring human migraine mutations cause synapse hyperexcitability that can be suppressed by inhibition of a Ca2+ store release pathway.
Brusich DJ, Spring AM, James TD, Yeates CJ, Helms TH, Frank CA. Brusich DJ, et al. PLoS Genet. 2018 Aug 6;14(8):e1007577. doi: 10.1371/journal.pgen.1007577. eCollection 2018 Aug. PLoS Genet. 2018. PMID: 30080864 Free PMC article. - Up-regulation of Ca2+/CaMKII/CREB signaling in salicylate-induced tinnitus in rats.
Zhao J, Wang B, Wang X, Shang X. Zhao J, et al. Mol Cell Biochem. 2018 Nov;448(1-2):71-76. doi: 10.1007/s11010-018-3314-z. Epub 2018 Feb 9. Mol Cell Biochem. 2018. PMID: 29427172 - The Ih Channel Gene Promotes Synaptic Transmission and Coordinated Movement in Drosophila melanogaster.
Hegle AP, Frank CA, Berndt A, Klose M, Allan DW, Accili EA. Hegle AP, et al. Front Mol Neurosci. 2017 Feb 24;10:41. doi: 10.3389/fnmol.2017.00041. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28286469 Free PMC article. - Homeostatic plasticity and burst activity are mediated by hyperpolarization-activated cation currents and T-type calcium channels in neuronal cultures.
Rátkai A, Tárnok K, Aouad HE, Micska B, Schlett K, Szücs A. Rátkai A, et al. Sci Rep. 2021 Feb 5;11(1):3236. doi: 10.1038/s41598-021-82775-3. Sci Rep. 2021. PMID: 33547341 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources