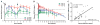Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus - PubMed (original) (raw)
. 2014 Mar 7;343(6175):1151-4.
doi: 10.1126/science.1248707. Epub 2014 Mar 4.
William R Spreen, Hiroshi Mohri, Lee Moss, Susan Ford, Agegnehu Gettie, Kasi Russell-Lodrigue, Rudolf P Bohm, Cecilia Cheng-Mayer, Zhi Hong, Martin Markowitz, David D Ho
Affiliations
- PMID: 24594934
- PMCID: PMC4308974
- DOI: 10.1126/science.1248707
Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus
Chasity D Andrews et al. Science. 2014.
Abstract
GSK1265744 (GSK744) is an integrase strand-transfer inhibitor that has been formulated as a long-acting (LA) injectable suitable for monthly to quarterly clinical administration. GSK744 LA was administered at two time points 4 weeks apart beginning 1 week before virus administration, and macaques were challenged weekly for 8 weeks. GSK744 LA, at plasma concentrations achievable with quarterly injections in humans, protected all animals against repeated low-dose challenges. In a second experiment, macaques were given GSK744 LA 1 week before virus administration and challenged repeatedly until infection occurred. Protection decreased over time and correlated with the plasma drug levels. With a quarterly dosing schedule in humans, our results suggest that GSK744 LA could potentially decrease adherence problems associated with daily preexposure prophylaxis (PrEP).
Figures
Fig. 1. GSK744 LA PK profile in rhesus macaques and humans
(A) GSK744 LA PKs were evaluated in rhesus macaques (n = 8) after intramuscular injections of 10 mg/kg into quadriceps as a single injection, 30 mg/kg (n = 4, two injections of 15 mg/kg) as a split injection, or 50 mg/kg (n = 8, four of 12.5 mg/kg) as a split dose with four injections, two per muscle. GSK744 LA was administered on weeks 0 and 4 (black arrows). (B) GSK744 LA single-dose PKs were evaluated in a phase 1 study, and the results were adapted here for comparison (16, 21). GSK744 LA was administered by intramuscular gluteal injection of 200 (n = 6), 400 (n = 14), or 800 (n = 6, two of 400 mg) mg to healthy human participants. Plasma GSK744 concentrations were assessed by liquid chromatography–mass spectrometry (HPLC-MS/MS) with a limit of quantitation (LOQ) >0.01 μg/ml. Means ± SDs are shown. Dotted and dashed horizontal lines represent 1× and 4× PAIC90, respectively. (C) Rectal tissue distribution of GSK744 was evaluated in macaques dosed with 10 or 30 mg/kg of GSK744 LA. The drug concentration from pinch mucosal biopsies was assessed each week in a subset (n = 4) of animals injected with 10 mg/kg. GSK744 concentration was assessed in all animals (n = 4) treated with 30 mg/kg of GSK744 LA at weeks 2, 4, and 7. Tissue concentrations were assessed by HPLC-MS/MS with LOQ > 0.05 μg/g. Each symbol represents the simultaneous plasma and rectal tissue concentrations of an individual macaque.
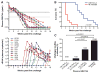
Fig. 2. Monthly injection of GSK744 LA protects macaques against repeated SHIV exposure
(A) Study design. Eight male macaques were injected IM in the quadriceps with 50 mg/kg (four of 12.5 mg/kg) of GSK744 LA at two time points, week –1 and 3. An additional eight male macaques were untreated and served as controls. All animals were challenged IR each week with 50 TCID50 of SHIV162P3 for up to eight exposures or until infection occurred. GSK744 LA–treated macaques were necropsied at week 19 for evaluation of proviral DNA in multiple tissues. (B) Kaplan-Meier plot of treated and untreated macaques remaining aviremic after serial SHIV challenges. (C) Viral loads of individual untreated control macaques. Dotted line represents the LOQ, >40 SHIV RNA copies per milliliter of plasma. (D) Plasma GSK744 concentrations in individual macaques throughout the course of study. Dotted and dashed horizontal lines represent 1× and 4× PAIC90, respectively. LOQ > 0.01 μg/ml.
Fig. 3. Plasma GSK744 levels that protected against repeated SHIV exposures
Twelve male macaques were injected IM with GSK744 LA at 50 mg/kg (four of 12.5 mg/kg) 1 week before the first virus exposure. Four macaques remained untreated as controls. All animals were challenged IR each week with 50 TCID50 of SHIV162P3 until infection was confirmed. (A) (Top) Plasma GSK744 concentrations from individual macaques. Open symbols correspond to first detection of viral RNA. Dotted and dashed horizontal lines represent 1× and 4× PAIC90, respectively. LOQ > 0.01 μg/ml. (Bottom) Viral loads of GSK744 LA–treated macaques. Dotted line represents the LOQ at >40 SHIV RNA copies per milliliter of plasma. (B) Kaplan-Meier plot demonstrating the delay of infection in GSK744 LA–treated macaques (n = 12) relative to untreated control macaques (n = 12; 4 from the current experiment and 8 from the first experiment). (C) Rate of SHIV infection within various ranges of GSK744 concentrations in plasma. The numbers above the bars represent the number of infections/number of challenges within a plasma concentration range. Plasma concentrations >3× PAIC90 yielded 100% protection, whereas concentrations >1× PAIC90 resulted in an infection rate of 1.2% (0/59 and 1/22). Compared with a 46.2% (12/26) infection rate in the placebo macaques, one can calculate a protective efficacy of ~97% in macaques with drug concentrations >1× PAIC90.
Comment in
- Virology. A bid to thwart HIV with shot of long-lasting drug.
Cohen J. Cohen J. Science. 2014 Mar 7;343(6175):1067. doi: 10.1126/science.343.6175.1067. Science. 2014. PMID: 24604173 No abstract available.
Similar articles
- A long-acting integrase inhibitor protects female macaques from repeated high-dose intravaginal SHIV challenge.
Andrews CD, Yueh YL, Spreen WR, St Bernard L, Boente-Carrera M, Rodriguez K, Gettie A, Russell-Lodrigue K, Blanchard J, Ford S, Mohri H, Cheng-Mayer C, Hong Z, Ho DD, Markowitz M. Andrews CD, et al. Sci Transl Med. 2015 Jan 14;7(270):270ra4. doi: 10.1126/scitranslmed.3010298. Sci Transl Med. 2015. PMID: 25589630 Free PMC article. - The long-acting integrase inhibitor GSK744 protects macaques from repeated intravaginal SHIV challenge.
Radzio J, Spreen W, Yueh YL, Mitchell J, Jenkins L, García-Lerma JG, Heneine W. Radzio J, et al. Sci Transl Med. 2015 Jan 14;7(270):270ra5. doi: 10.1126/scitranslmed.3010297. Sci Transl Med. 2015. PMID: 25589631 - Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251.
Andrews CD, Bernard LS, Poon AY, Mohri H, Gettie N, Spreen WR, Gettie A, Russell-Lodrigue K, Blanchard J, Hong Z, Ho DD, Markowitz M. Andrews CD, et al. AIDS. 2017 Feb 20;31(4):461-467. doi: 10.1097/QAD.0000000000001343. AIDS. 2017. PMID: 27902508 Free PMC article. - Islatravir for the treatment and prevention of infection with the human immunodeficiency virus type 1.
Markowitz M, Grobler JA. Markowitz M, et al. Curr Opin HIV AIDS. 2020 Jan;15(1):27-32. doi: 10.1097/COH.0000000000000599. Curr Opin HIV AIDS. 2020. PMID: 31658118 Review. - Cabotegravir long-acting for HIV-1 prevention.
Andrews CD, Heneine W. Andrews CD, et al. Curr Opin HIV AIDS. 2015 Jul;10(4):258-63. doi: 10.1097/COH.0000000000000161. Curr Opin HIV AIDS. 2015. PMID: 26049951 Review.
Cited by
- Quantum Mechanical-Based Stability Evaluation of Crystal Structures for HIV-Targeted Drug Cabotegravir.
Han Y, Luo H, Lu Q, Liu Z, Liu J, Zhang J, Wei Z, Li J. Han Y, et al. Molecules. 2021 Nov 26;26(23):7178. doi: 10.3390/molecules26237178. Molecules. 2021. PMID: 34885762 Free PMC article. - Approved Antiviral Drugs over the Past 50 Years.
De Clercq E, Li G. De Clercq E, et al. Clin Microbiol Rev. 2016 Jul;29(3):695-747. doi: 10.1128/CMR.00102-15. Clin Microbiol Rev. 2016. PMID: 27281742 Free PMC article. Review. - Synthesis of a long acting nanoformulated emtricitabine ProTide.
Soni D, Bade AN, Gautam N, Herskovitz J, Ibrahim IM, Smith N, Wojtkiewicz MS, Dyavar Shetty BL, Alnouti Y, McMillan J, Gendelman HE, Edagwa BJ. Soni D, et al. Biomaterials. 2019 Nov;222:119441. doi: 10.1016/j.biomaterials.2019.119441. Epub 2019 Aug 20. Biomaterials. 2019. PMID: 31472458 Free PMC article. - Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial.
Landovitz RJ, Li S, Grinsztejn B, Dawood H, Liu AY, Magnus M, Hosseinipour MC, Panchia R, Cottle L, Chau G, Richardson P, Marzinke MA, Hendrix CW, Eshleman SH, Zhang Y, Tolley E, Sugarman J, Kofron R, Adeyeye A, Burns D, Rinehart AR, Margolis D, Spreen WR, Cohen MS, McCauley M, Eron JJ. Landovitz RJ, et al. PLoS Med. 2018 Nov 8;15(11):e1002690. doi: 10.1371/journal.pmed.1002690. eCollection 2018 Nov. PLoS Med. 2018. PMID: 30408115 Free PMC article. Clinical Trial. - Tackling HIV and AIDS: contributions by non-human primate models.
Van Rompay KKA. Van Rompay KKA. Lab Anim (NY). 2017 May 22;46(6):259-270. doi: 10.1038/laban.1279. Lab Anim (NY). 2017. PMID: 28530684
References
- UNAIDS. 2013 UNAIDS Report on the Global AIDS Epidemic. 2013 www.unaids.org/en/resources/documents/2013/name,85053,en.asp.
- Thigpen MC, et al. N Engl J Med. 2012;367:423–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R0-AI100724/AI/NIAID NIH HHS/United States
- P51 OD011104/OD/NIH HHS/United States
- 2P51-OD11104-52/OD/NIH HHS/United States
- 1DP1-DA033263/DA/NIDA NIH HHS/United States
- DP1 DA033263/DA/NIDA NIH HHS/United States
- R01 AI100724/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous