Decreased anxiety-like behavior and Gαq/11-dependent responses in the amygdala of mice lacking TRPC4 channels - PubMed (original) (raw)
Decreased anxiety-like behavior and Gαq/11-dependent responses in the amygdala of mice lacking TRPC4 channels
Antonio Riccio et al. J Neurosci. 2014.
Abstract
Transient receptor potential (TRP) channels are abundant in the brain where they regulate transmission of sensory signals. The expression patterns of different TRPC subunits (TRPC1, 4, and 5) are consistent with their potential role in fear-related behaviors. Accordingly, we found recently that mutant mice lacking a specific TRP channel subunit, TRPC5, exhibited decreased innate fear responses. Both TRPC5 and another member of the same subfamily, TRPC4, form heteromeric complexes with the TRPC1 subunit (TRPC1/5 and TRPC1/4, respectively). As TRP channels with specific subunit compositions may have different functional properties, we hypothesized that fear-related behaviors could be differentially controlled by TRPCs with distinct subunit arrangements. In this study, we focused on the analysis of mutant mice lacking the TRPC4 subunit, which, as we confirmed in experiments on control mice, is expressed in brain areas implicated in the control of fear and anxiety. In behavioral experiments, we found that constitutive ablation of TRPC4 was associated with diminished anxiety levels (innate fear). Furthermore, knockdown of TRPC4 protein in the lateral amygdala via lentiviral-mediated gene delivery of RNAi mimicked the behavioral phenotype of constitutive TRPC4-null (TRPC4(-/-)) mouse. Recordings in brain slices demonstrated that these behavioral modifications could stem from the lack of TRPC4 potentiation in neurons in the lateral nucleus of the amygdala through two Gαq/11 protein-coupled signaling pathways, activated via Group I metabotropic glutamate receptors and cholecystokinin 2 receptors, respectively. Thus, TRPC4 and the structurally and functionally related subunit, TRPC5, may both contribute to the mechanisms underlying regulation of innate fear responses.
Keywords: TRP channel; TRPC4; amygdala; anxiety; cholecystokinin 4; fear.
Figures
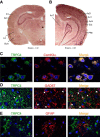
Figure 1.
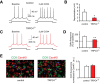
TRPC4 expression in adult mouse brain. A, B, In situ hybridization of _TRPC4_-mRNA in amygdala, hippocampus, somatosensory cortex, auditory thalamus, and auditory cortex. BLA, amygdala basolateral nucleus; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; AuT, auditory thalamus; AuD, secondary auditory cortex, dorsal; Au1, primary auditory cortex; AuV, secondary auditory cortex, ventral; Ect, ectorhinal cortex; PRh, perirhinal cortex. Scale bar, 1 mm. C, TRPC4 (left) and CaMKIIα (middle; a marker of pyramidal neurons) colocalize in the LA (right). D, Cells expressing TRPC4 (red) and GFAP (green; a marker of glial cells) do not colocalize in the LA. E, Cells expressing TRPC5 (red) and GAD67 (green; a marker of interneurons) do not colocalize in the LA. Scale bars: C–E, 10 μm.
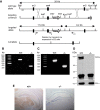
Figure 2.
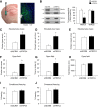
Generation and confirmation of _TRPC4_−/− mouse. A, Targeting strategy for the disruption of the TRPC4 gene. After homologous recombination, deletion of exon 4 region was catalyzed by _Cre_-recombinase in ES cells. B, Targeting of the TRPC4 locus is confirmed by PCR analysis of tail genomic DNA. C, RT-PCR analysis of whole-brain mRNA from control and _TRPC4_−/− littermates confirms the absence of exon 4. D, IP of TRPC4 protein in brain microsomes from control and _TRPC4_−/− mice reveals loss of TRPC4 protein in _TRPC4_−/− mice (top). Western blotting of NKA-α confirms equal protein loading in control and _TRPC4_−/− mice (bottom). E, Immunohistochemical staining of brain sections from control and _TRPC4_−/− littermates reveals selective loss of TRPC4 expression in mutant mice. Scale bar, 1 mm.
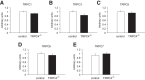
Figure 3.
qRT-PCR determination of TRPC mRNA levels in the brain. A–E, Whole-brain mRNA levels of TRPC1, TRPC3, TRPC5, TRPC6, and TRPC7 were not different between control and _TRPC4_−/− littermates. Data are expressed as arbitrary units normalized to β-actin to correct for RNA quantity and integrity and presented as mean ± SEM for triplicate reverse transcription reactions from two RNA pools (triplicate data from each of 2 control and 2 KO mice).
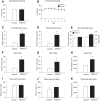
Figure 4.
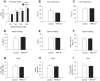
_TRPC4_−/− mice exhibit an anxiolytic-like phenotype. A, B, No differences in the percentage of conditioned freezing (A) or contextual freezing (B) were observed between control and null mice 24 h post training (n = 10 mice per each group). C–E, Elevated plus maze experiments (n = 10 mice per each group). TRPC4−/− mice entered the open arms more commonly (C) and spent more time in the open arms (D), but did not differ in the closed-arm entries (E, left) or total entries (E, right). F–H, Open field test (100 lux). TRPC4−/− mice entered the center more frequently (F), spent significantly more time in the center of the arena (G), and were more active (H). Data from 10 control and 9 null mice. I–K, In open field tests under red light (nonanxiogenic) conditions, there were no differences between control (n = 10) and null (n = 10) mice in exploration of the center of the arena for entries into the center (I), time spent in the center (J), or general exploratory activity (K). Results are shown as mean ± SEM.
Figure 5.
Lack of generalized behavioral deficits in _TRPC4_−/− mice. A, No significant differences were observed between control (empty bars) and null mice (filled bars) in acoustic startle responses to auditory stimuli at 90, 95, 100, and 105 dB (n = 10 mice per group). Responses were calculated as the maximum force in Newtons (N) based on the highest absolute value (during extension or retraction of the legs). B, C, Tail suspension test. When suspended by the tail for a 6 min test session, mice in both groups (n = 10 mice per each group) assumed an immobile posture within 40–60 s (B) and remained immobile for the times shown in C. There were no significant differences between genotypes. D–F, Beam walking test. Control and null mice (n = 10 mice per each group) did not show significant differences in percentage of foot slips (errors) on a narrow (4 mm wide) balance beam (D), number of steps (E), or mean crossing times (F). G–I, Gait analyses. No significant differences between groups were observed in paw angle variability (G), ataxia coefficient (H), and stride length variability (I) parameters. Results are shown as mean ± SEM.
Figure 6.
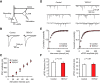
Basal synaptic transmission and LTP are normal at the cortico-amygdala and thalamo-amygdala synapses in _TRPC4_−/− mice. A, Schematic representation of a brain slice preparation containing the amygdala that shows the position of the stimulation electrode (Sthalamic, Scortical) and recording (R) pipette. EC, external capsule. B, Responses of LA neurons in slices from control and _TRPC4_−/− mice to a prolonged current injection (200 pA, 500 ms) recorded in current-clamp mode. C, Number of APs in LA neurons evoked by current injections of increasing intensity in slices from control (open symbols) and _TRPC4_−/− (filled symbols) mice, recorded as in B. D, Synaptic input–output curves for the EPSCs recorded at the cortico-LA synapses. The EPSCs were recorded under voltage-clamp conditions at a holding potential of –70 mV. E, Spike timing-dependent LTP of the cortico-amygdala EPSPs recorded in LA neurons in slices from control and mutant mice. Insets are averages of 10 EPSPs recorded before and 35 min after induction. F, Summary of LTP experiments in cortical input to the LA. G, Synaptic input–output curves for the EPSCs recorded at the thalamo-LA synapses. The EPSCs were recorded under voltage-clamp conditions at a holding potential of –70 mV. H, LTP in thalamic input to the LA. Insets show the average of 10 EPSPs recorded before and 35 min after induction. I, Summary of LTP experiments in thalamic input to the LA. Results are shown as mean ± SEM.
Figure 7.
TRPC4 ablation had no effect on parameters of glutamatergic mEPSCs. A, mEPSCs recorded in LA neurons in slices from control (left) and _TRPC4_−/− mice (right). B, Cumulative interevent interval histograms of mEPSCs recorded in LA neurons in slices from control and _TRPC4_−/− mice. C, Summary plot showing averaged mEPSC frequency data. D, Cumulative amplitude histograms of mEPSCs recorded in LA neurons in slices from control and _TRPC4_−/− mice. E, Summary plot showing mean peak amplitude data from same recordings as in D. Results are shown as mean ± SEM.
Figure 8.
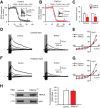
GABAA receptor-mediated synaptic responses in LA neurons are normal in _TRPC4_−/− mice. A, Schematic representation of the neuronal circuit for fast GABAergic inhibition in the LA and the experimental design. LA/PN, principal neuron in the LA; LA/IN, interneurons in the LA. B, IPSCs (average of 10 traces), which were evoked in LA neurons by stimulation pulses of two different intensities in slices from control and _TRPC4_−/− mice. The stimulation electrode was placed within the LA, and the external solution contained CNQX (20 μ
m
). The IPSCs were recorded with a chloride-based intrapipette solution (Shumyatsky et al., 2005). C, Input–output relations for evoked GABAAR IPSCs in slices from control and _TRPC4_−/− mice. D, Representative recordings of sIPSCs in LA neurons at –70 mV in slices from control and _TRPC4_−/− mice. E, Cumulative interevent interval (left) and amplitude (right) histograms of sIPSCs recorded in slices from control and _TRPC4_−/− mice. F, Summary of sIPSCs parameters for data in E. The graph shows averaged sIPSC frequency (left) and mean peak amplitude (right). Results are shown as mean ± SEM.
Figure 9.
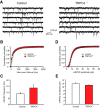
mGluR-EPSCs in LA neurons are suppressed in _TRPC4_−/− mice. A, Synaptic responses in cortical input to the LA neuron in a slice from a control mouse evoked by trains of high-frequency stimulation before and during addition of CNQX (AMPA receptor antagonist; 20 μ
m
), NMDA receptor antagonists,
d
-APV (50 μ
m
), and MK-801 (10 μm) and GABABR antagonist CGP 35348 (300 μ
m
) recorded as described previously (Riccio et al., 2009). Stimulation trains consisted of 10 pulses at 100 Hz which were delivered once every 30 s. Inset shows synaptic responses recorded in current-clamp mode before (1) and after (2) the addition of antagonists to the external medium. The dashed line indicates the time point where the EPSP amplitude was measured. B, The experiment was identical to A, but the recording was obtained from a _TRPC4_−/− mouse. C, Summary data for the experiments as in A and B, performed in both cortical and thalamic inputs to the LA. The amplitude of the residual component of the EPSP in the presence of antagonists was smaller in both pathways in slices from _TRPC4_−/− mice compared with control littermates. D, EPSCs in cortical input recorded in voltage-clamp mode at holding potentials ranging from –100 mV to +40 mV in slices from control (left) and _TRPC4_−/− (right) mice in the presence of the antagonists (as in A). E, Current–voltage (I–V) plots of the peak current in cortical input (as in D) in slices from control and _TRPC4_−/− mice. F, EPSCs in thalamic input recorded in voltage-clamp mode at holding potentials ranging from –100 mV to +40 mV in slices from control (left) and _TRPC4_−/− (right) mice in the presence of the antagonists (as in A). G, Current–voltage (I–V) plots of the peak current in thalamic input (as in F) in slices from control and _TRPC4_−/− mice. H, Left, Representative immunoblot shows similar amounts of TRPC5 protein in brain microsomes extracted from control or _TRPC4_−/− littermates. Western blotting of Na+-K+-ATPase indicates equal protein loading (bottom). Right, Quantification of three different blots. Results are shown as mean ± SEM.
Figure 10.
CCK-mediated increase in spike firing in LA neurons is diminished in _TRPC4_−/− mice. A, Spikes evoked in LA neurons by current injection (150 pA) recorded in current-clamp mode under baseline conditions and in the presence of 3 μ
m
CCK4 in a slice from a control mouse. B, Summary plot showing CCK4-induced depolarization in LA neurons in slices from control and mutant mice. C, Spikes evoked in LA neuron under baseline conditions and in the presence of 3 μ
m
CCK4 in a slice from a _TRPC4_−/− mouse. D, The percentage increase in spike frequency in the presence of CCK4 relative to the baseline frequency (taken as 100%) in slices from control and null mice. E, Left, Fluorescence double labeling for CCKergic fibers (green) and CaMKIIα (red) reveals no differences in innervation of the LA by CCK-containing fibers in brain sections from control and _TRPC4_−/− littermates. Right, Mean CCK fluorescence after normalization to CaMKIIα fluorescence from six images per group taken from different stainings (two images from each of 3 control and 3 KO mice). Scale bar, 10 μm. Results are shown as mean ± SEM.
Figure 11.
Knockdown of TRPC4 in the LA. A, Left, An image illustrating the injection experiments. Right, a microscopic image showing expression of LV-shTRPC4-GFP in the LA (green). Scale bar, 50 μm. B, Left, Representative Western blots showing TRPC4 knockdown and TRPC5 expression in the LA. Right, Analysis of TRPC4 and TRPC5 in LA homogenates taken 4 weeks following intra-LA infusion of LV-shTRPC4-GFP (n = 5) or LV-SCRM-GFP (n = 5). Protein levels were normalized to GAPDH). C–E, Elevated plus maze experiments (n = 10 mice per a group). Mice with TRPC4 knockdown exhibited an increased number of entries into the open arms (C), spent more time in the open arms (D), but showed no differences in closed-arm entries (E). F–H, Open field test (100 lux; n = 10 mice per group). TRPC4 knockdown mice entered the center of the arena more frequently (F) and spent more time in it (G), and travel the same distance as control mice (H). I, J, No differences in freezing responses (n = 10 mice per a group) were observed in fear conditioning (I) or contextual fear tests (J). Results are shown as mean ± SEM.
Similar articles
- Treatment with HC-070, a potent inhibitor of TRPC4 and TRPC5, leads to anxiolytic and antidepressant effects in mice.
Just S, Chenard BL, Ceci A, Strassmaier T, Chong JA, Blair NT, Gallaschun RJ, Del Camino D, Cantin S, D'Amours M, Eickmeier C, Fanger CM, Hecker C, Hessler DP, Hengerer B, Kroker KS, Malekiani S, Mihalek R, McLaughlin J, Rast G, Witek J, Sauer A, Pryce CR, Moran MM. Just S, et al. PLoS One. 2018 Jan 31;13(1):e0191225. doi: 10.1371/journal.pone.0191225. eCollection 2018. PLoS One. 2018. PMID: 29385160 Free PMC article. - Essential role for TRPC5 in amygdala function and fear-related behavior.
Riccio A, Li Y, Moon J, Kim KS, Smith KS, Rudolph U, Gapon S, Yao GL, Tsvetkov E, Rodig SJ, Van't Veer A, Meloni EG, Carlezon WA Jr, Bolshakov VY, Clapham DE. Riccio A, et al. Cell. 2009 May 15;137(4):761-72. doi: 10.1016/j.cell.2009.03.039. Cell. 2009. PMID: 19450521 Free PMC article. - Heteromeric channels formed by TRPC1, TRPC4 and TRPC5 define hippocampal synaptic transmission and working memory.
Bröker-Lai J, Kollewe A, Schindeldecker B, Pohle J, Nguyen Chi V, Mathar I, Guzman R, Schwarz Y, Lai A, Weißgerber P, Schwegler H, Dietrich A, Both M, Sprengel R, Draguhn A, Köhr G, Fakler B, Flockerzi V, Bruns D, Freichel M. Bröker-Lai J, et al. EMBO J. 2017 Sep 15;36(18):2770-2789. doi: 10.15252/embj.201696369. Epub 2017 Aug 8. EMBO J. 2017. PMID: 28790178 Free PMC article. - TRPC1 as a negative regulator for TRPC4 and TRPC5 channels.
Kim J, Ko J, Myeong J, Kwak M, Hong C, So I. Kim J, et al. Pflugers Arch. 2019 Aug;471(8):1045-1053. doi: 10.1007/s00424-019-02289-w. Epub 2019 Jun 20. Pflugers Arch. 2019. PMID: 31222490 Review. - TRPC5.
Zholos AV. Zholos AV. Handb Exp Pharmacol. 2014;222:129-56. doi: 10.1007/978-3-642-54215-2_6. Handb Exp Pharmacol. 2014. PMID: 24756705 Review.
Cited by
- TRPC4 as a coincident detector of Gi/o and Gq/11 signaling: mechanisms and pathophysiological implications.
Jeon J, Tian JB, Zhu MX. Jeon J, et al. Curr Opin Physiol. 2020 Oct;17:34-41. doi: 10.1016/j.cophys.2020.06.008. Epub 2020 Jul 2. Curr Opin Physiol. 2020. PMID: 32851198 Free PMC article. - Cryo-EM structure of TRPC5 at 2.8-Å resolution reveals unique and conserved structural elements essential for channel function.
Duan J, Li J, Chen GL, Ge Y, Liu J, Xie K, Peng X, Zhou W, Zhong J, Zhang Y, Xu J, Xue C, Liang B, Zhu L, Liu W, Zhang C, Tian XL, Wang J, Clapham DE, Zeng B, Li Z, Zhang J. Duan J, et al. Sci Adv. 2019 Jul 24;5(7):eaaw7935. doi: 10.1126/sciadv.aaw7935. eCollection 2019 Jul. Sci Adv. 2019. PMID: 31355338 Free PMC article. - TRPC4 and GIRK channels underlie neuronal coding of firing patterns that reflect Gq/11-Gi/o coincidence signals of variable strengths.
Tian JB, Yang J, Joslin WC, Flockerzi V, Prescott SA, Birnbaumer L, Zhu MX. Tian JB, et al. Proc Natl Acad Sci U S A. 2022 May 17;119(20):e2120870119. doi: 10.1073/pnas.2120870119. Epub 2022 May 11. Proc Natl Acad Sci U S A. 2022. PMID: 35544691 Free PMC article. - Psychiatric Disorders and TRP Channels: Focus on Psychotropic Drugs.
Nazıroğlu M, Demirdaş A. Nazıroğlu M, et al. Curr Neuropharmacol. 2015;13(2):248-57. doi: 10.2174/1570159x13666150304001606. Curr Neuropharmacol. 2015. PMID: 26411768 Free PMC article. Review. - Distribution of type I corticotropin-releasing factor (CRF1) receptors on GABAergic neurons within the basolateral amygdala.
Calakos KC, Blackman D, Schulz AM, Bauer EP. Calakos KC, et al. Synapse. 2017 Apr;71(4):10.1002/syn.21953. doi: 10.1002/syn.21953. Epub 2017 Feb 20. Synapse. 2017. PMID: 27997737 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- T32 HL007572/HL/NHLBI NIH HHS/United States
- MH090293/MH/NIMH NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- R01 MH090293/MH/NIMH NIH HHS/United States
- MH090464/MH/NIMH NIH HHS/United States
- R01 MH090464/MH/NIMH NIH HHS/United States
- P30-HD18655/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous