The role of CRISPR-Cas systems in virulence of pathogenic bacteria - PubMed (original) (raw)
Review
The role of CRISPR-Cas systems in virulence of pathogenic bacteria
Rogier Louwen et al. Microbiol Mol Biol Rev. 2014 Mar.
Abstract
Clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated (Cas) genes are present in many bacterial and archaeal genomes. Since the discovery of the typical CRISPR loci in the 1980s, well before their physiological role was revealed, their variable sequences have been used as a complementary typing tool in diagnostic, epidemiologic, and evolutionary analyses of prokaryotic strains. The discovery that CRISPR spacers are often identical to sequence fragments of mobile genetic elements was a major breakthrough that eventually led to the elucidation of CRISPR-Cas as an adaptive immunity system. Key elements of this unique prokaryotic defense system are small CRISPR RNAs that guide nucleases to complementary target nucleic acids of invading viruses and plasmids, generally followed by the degradation of the invader. In addition, several recent studies have pointed at direct links of CRISPR-Cas to regulation of a range of stress-related phenomena. An interesting example concerns a pathogenic bacterium that possesses a CRISPR-associated ribonucleoprotein complex that may play a dual role in defense and/or virulence. In this review, we describe recently reported cases of potential involvement of CRISPR-Cas systems in bacterial stress responses in general and bacterial virulence in particular.
Figures
FIG 1
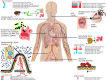
Overview of expression of cas genes in human-associated bacteria that occupy different host niches. The heat maps indicate which cas genes are induced (shades of red) or repressed (shades of blue) during bacterial responses to changes in the environment. Details are given in the main text. The overview shows that modulation of cas gene expression occurs in diverse Gram-positive and Gram-negative bacteria that together occupy very diverse niches throughout the human body. For F. novicida, adaptation of gene expression in macrophages depends on Cas9, tracRNA, and possibly also scaRNA, which together inhibit expression of an immunogenic lipoprotein (shown in green) (22). All bacteria depicted in this figure possess Cas9, and scaRNA production has been predicted for F. novicida, C. jejuni, L. monocytogenes, and N. meningitidis (22), suggesting that a role of Cas9 in regulation of bacterial gene expression may be more widespread.
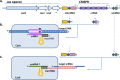
FIG 2
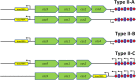
Overview of the three type II CRISPR-Cas subtypes. All three subtypes share a conserved set of cas genes: cas1, cas2, and cas9. Type II-A has an additional csn2 gene, and type II-B has an additional cas4 gene (21). Type II-C does not feature an additional cas gene beyond the three conserved cas genes (122). All subtypes feature a small _trans_-encoded RNA called _trans_-activating CRISPR RNA (tracrRNA); type II-C displays variation in the location of tracrRNA. cas9, cas1, and cas2 are indicated with green arrows, and tracrRNA is shown with yellow boxes. Transcription start sites are shown as black arrows upstream of the repeats (red diamonds) and spacers (purple squares) in type II-A (e.g., in Streptococcus spp.) and -B (e.g., in Legionella pneumophila) CRISPR loci, or within each spacer in the case of the minimal type II-C CRISPR systems of Neisseria meningitidis (upper) and Campylobacter jejuni (lower) (122).
FIG 3
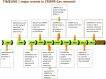
Overview of the most important discoveries in CRISPR-Cas research. The original papers describing the major findings are discussed and cited in the main text.
FIG 4
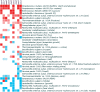
Heat map showing expression of cas genes in human-associated bacteria that occupy different host niches. The heat map indicates which cas genes are induced (shades of red) or repressed (shades of blue) during bacterial responses to changes in the environment. All gene expression data displayed in this figure have been published, are publicly available in the MicrobesOnline (
http://www.microbesonline.org/
) and NCBI Entrez (
http://www.ncbi.nlm.nih.gov/gene/
) gene expression databases, and are further discussed in this review.
FIG 5
Dual function of type II CRISPR-Cas systems. (A) Genomic locus of type II CRISPR-Cas system. The cas operon consists of at least three genes (cas9, cas1, and cas2). A fourth gene (*) is present in type II-A (csn2) and II-B (cas4) systems but not in type II-C systems (122). Adjacent to the cas operon, the CRISPR locus is present (dark purple diamonds indicate repeats, and bright purple squares indicate the spacers), as well as the _trans_-encoded CRISPR RNA (tracrRNA) gene and possibly the recently proposed scaRNA gene (22). The order and orientation of the CRISPR and the genes vary in different genomes. (B) A role in defense against DNAs of invading genetic elements is well established (11), in which processed crRNA and a short version of the tracrRNA (most likely resulting from processing of a longer tracrRNA transcript or transcription from a second promoter; see panel A) eventually are responsible for interaction with target DNA. Eventually, both DNA strands are cleaved at the active sites of Cas9 (red triangles) (20, 129, 130). (C) A distinct role of Cas9 in virulence has been suggested (30), and a molecular basis for how Cas9 can codetermine virulence has been revealed (22): a long version of the tracrRNA shares significant homology with a target transcript, resulting in silencing and probably degradation of this transcript. Involvement of another small CRISPR-associated RNA (scaRNA) has been proposed; if indeed important, this scaRNA may be involved in stabilizing the interaction of the tracrRNA in the Cas9 complex.
Similar articles
- CRISPR-Cas systems: beyond adaptive immunity.
Westra ER, Buckling A, Fineran PC. Westra ER, et al. Nat Rev Microbiol. 2014 May;12(5):317-26. doi: 10.1038/nrmicro3241. Epub 2014 Apr 7. Nat Rev Microbiol. 2014. PMID: 24704746 Review. - Survey of clustered regularly interspaced short palindromic repeats and their associated Cas proteins (CRISPR/Cas) systems in multiple sequenced strains of Klebsiella pneumoniae.
Ostria-Hernández ML, Sánchez-Vallejo CJ, Ibarra JA, Castro-Escarpulli G. Ostria-Hernández ML, et al. BMC Res Notes. 2015 Aug 4;8:332. doi: 10.1186/s13104-015-1285-7. BMC Res Notes. 2015. PMID: 26238567 Free PMC article. - The CRISPR Spacer Space Is Dominated by Sequences from Species-Specific Mobilomes.
Shmakov SA, Sitnik V, Makarova KS, Wolf YI, Severinov KV, Koonin EV. Shmakov SA, et al. mBio. 2017 Sep 19;8(5):e01397-17. doi: 10.1128/mBio.01397-17. mBio. 2017. PMID: 28928211 Free PMC article. - Clustered regularly interspaced short palindromic repeats (CRISPRs): the hallmark of an ingenious antiviral defense mechanism in prokaryotes.
Al-Attar S, Westra ER, van der Oost J, Brouns SJ. Al-Attar S, et al. Biol Chem. 2011 Apr;392(4):277-89. doi: 10.1515/BC.2011.042. Epub 2011 Feb 7. Biol Chem. 2011. PMID: 21294681 Review. - Viral diversity threshold for adaptive immunity in prokaryotes.
Weinberger AD, Wolf YI, Lobkovsky AE, Gilmore MS, Koonin EV. Weinberger AD, et al. mBio. 2012 Dec 4;3(6):e00456-12. doi: 10.1128/mBio.00456-12. mBio. 2012. PMID: 23221803 Free PMC article.
Cited by
- Genomic population structure of Helicobacter pylori Shanghai isolates and identification of genomic features uniquely linked with pathogenicity.
Yang F, Zhang J, Wang S, Sun Z, Zhou J, Li F, Liu Y, Ding L, Liu Y, Chi W, Liu T, He Y, Xiang P, Bao Z, Olszewski MA, Zhao H, Zhang Y. Yang F, et al. Virulence. 2021 Dec;12(1):1258-1270. doi: 10.1080/21505594.2021.1920762. Virulence. 2021. PMID: 33904371 Free PMC article. - Imipenem represses CRISPR-Cas interference of DNA acquisition through H-NS stimulation in Klebsiella pneumoniae.
Lin TL, Pan YJ, Hsieh PF, Hsu CR, Wu MC, Wang JT. Lin TL, et al. Sci Rep. 2016 Aug 17;6:31644. doi: 10.1038/srep31644. Sci Rep. 2016. PMID: 27531594 Free PMC article. - CRISPR genotyping as complementary tool for epidemiological surveillance of Erwinia amylovora outbreaks.
Mendes RJ, Luz JP, Santos C, Tavares F. Mendes RJ, et al. PLoS One. 2021 Apr 16;16(4):e0250280. doi: 10.1371/journal.pone.0250280. eCollection 2021. PLoS One. 2021. PMID: 33861806 Free PMC article. - Proteomic analysis of the regulatory networks of ClpX in a model cyanobacterium Synechocystis sp. PCC 6803.
Zhang Y, Wang Y, Wei W, Wang M, Jia S, Yang M, Ge F. Zhang Y, et al. Front Plant Sci. 2022 Sep 29;13:994056. doi: 10.3389/fpls.2022.994056. eCollection 2022. Front Plant Sci. 2022. PMID: 36247581 Free PMC article. - Distribution, Diversity and Roles of CRISPR-Cas Systems in Human and Animal Pathogenic Streptococci.
Lemaire C, Le Gallou B, Lanotte P, Mereghetti L, Pastuszka A. Lemaire C, et al. Front Microbiol. 2022 Jan 31;13:828031. doi: 10.3389/fmicb.2022.828031. eCollection 2022. Front Microbiol. 2022. PMID: 35173702 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources