Inhibition of mammalian target of rapamycin improves neurobehavioral deficit and modulates immune response after intracerebral hemorrhage in rat - PubMed (original) (raw)
Inhibition of mammalian target of rapamycin improves neurobehavioral deficit and modulates immune response after intracerebral hemorrhage in rat
Qin Lu et al. J Neuroinflammation. 2014.
Abstract
Background: Mammalian target of rapamycin (mTOR), a serine/threonine kinase, regulates many processes, including cell growth and the immune response. mTOR is also dysregulated in several neurological diseases, such as traumatic brain injury (TBI), stroke, and neurodegenerative disease. However, the role of mTOR in intracerebral hemorrhage (ICH) remains unexplored. The aims of our study were to determine whether inhibiting mTOR signaling could affect the outcome after ICH and to investigate the possible underlying mechanism.
Methods: A rat ICH model was induced by intracerebral injection of collagenase IV into the striatum, and mTOR activation was inhibited by administration of rapamycin. mTOR signaling activation was determined by western blotting. Neurobehavioral deficit after ICH was determined by a set of modified Neurological Severity Scores (mNSS). The levels of CD4+CD25+Foxp3+ regulatory T cells (Tregs) and cytokines were examined using flow cytometry and ELISA, respectively.
Results: Our results demonstrated thatmTOR signaling was activated 30 minutes and returned to its basal level 1 day after ICH. Increased p-mTOR, which mean that mTOR signaling was activated, was predominantly located around the hematoma. Rapamycin treatment significantly improved the neurobehavioral deficit after ICH, increased the number of Tregs, increased levels of interleukin-10 and transforming growth factor-β and reduced interferon-γ both in peripheral blood and brain.
Conclusions: Our study suggests that mTOR improves ICH outcome and modulates immune response after ICH.
Figures
Figure 1
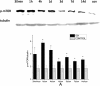
Mammalian target of rapamycin (mTOR) was activated after intracerebral hemorrhage (ICH). (A) Protein from the ipsilateral hemisphere was analyzed by western blotting using anti-p-mTOR (Ser 2448)., which was was greatly increased at 30 minutes after ICH and lasted up to 14 days after ICH. The level of p-mTOR was normalized to the level of tubulin. *_P<_0.05.
Figure 2
Increased mammalian target of rapamycin (mTOR) activation in the ipsilateral striatum after intracerebral hemorrhage (ICH). (A) Protein from the striatum was analyzed by western blotting using anti-p-mTOR (Ser 2448). The level of p-mTOR was normalized to the level of tubulin. The p-mTOR significantly increased at 30 minutes after ICH, and returned to basal level at 1 day after ICH. (B) The change in total mTOR was not significant after being normalized to tubulin. *_P<_0.05; **_P<_0.001.
Figure 3
Mammalian target of rapamycin (mTOR) activation in the ipsilateral cortex after intracerebral hemorrhage (ICH). The levels of both (A) p-mTOR and (B) total mTOR did not show significant changes after ICH (after normalization to tubulin).
Figure 4
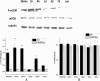
Increased p70S6 activation in the ipsilateral striatum after intracerebral hemorrhage (ICH). (A) Protein from the striatum was analyzed by western blotting using anti-p-p70S6 (Thr 389). The level of p-p70S6 was normalized to the level of tubulin. p-mTOR was significantly increased at 1 hour and returned to basal level at 1 day after ICH. (B) The change in total p70S6 was not significant after being normalized to tubulin. _*P<_0.05.
Figure 5
Rapamycin improves recovery of neurobehavioral function after intracerebral hemorrhage (ICH). The scores in both the ICH and rapamycin groups were similar before ICH. At 1 hour after ICH, rapamycin-treated groups were injected with rapamycin using different concentrations: 50, 150, 250, and 500 μg/kg. The behaviors were evaluated and compared at 1, 3, 7, and 14 days after ICH. We observed a significant functional recovery in rapamycin-treated groups compared with the ICH group. The mean modified Neurological Severity Score (mNSS) values ± SEM are depicted, *_P<_0.05; ** _P<_0.001 compared with ICH rats.
Figure 6
Rapamycin inhibited p-mammalian target of rapamycin (mTOR) in the striatum. (A) Compared with the intracerebral hemorrhage (ICH) group (4 h after ICH), significantly lower levels of p-mTOR were observed in the 150, 250, and 500 μg/kg treated groups while there was no significant change in the 50 μg/kg treated group. (B) The total mTOR level in rapamycin-treated groups was similar to that of the ICH group. Both p-mTOR and total mTOR were normalized to tubulin. *_P<_0.05; **_P<_0.001.
Figure 7
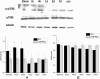
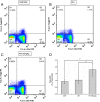
Rapamycin increased the level of regulatory T cells (Tregs) in the blood. Dot plots labeled with CD4 and Foxp3 show the blood lymphocytes derived from (A) control group, (B) intracerebral hemorrhage (ICH) group, and (C) 150 μg/kg rapamycin-treated group. (D) A statistical graph for the three groups. There were significant differences between the control group and rapamycin-treated groups, and between the ICH and rapamycin-treated groups. _*P<_0.05.
Figure 8
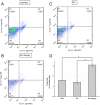
Rapamycin increased the level of regulatory T cells (Tregs) in the ipsilateral hemisphere. Dot plots labeled with CD4 and Foxp3 show the brain lymphocytes from (A) control group, (B) intracerebral hemorrhage (ICH) group, and (C) 150 μg/kg rapamycin-treated group. (D) A statistical graph for the three groups. There were significantly higher levels of Tregs in the rapamycin-treated group than the control and ICH groups. *_P<_0.001.
Figure 9
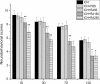
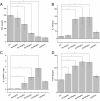
Levels of cytokines in the serum after rapamycin treatment. (A) The levels of interferon (IFN)-γ in each rapamycin-treated group were lower than the intracerebral hemorrhage (ICH) group. (B) Rapamycin-treated groups presented higher interleukin (IL)-10 expression than the ICH group. (C) The ratio of interleukin (IL)-10 to IFN-γ increased after treatment with rapamycin (150, 250, and 500 μg/kg, but not 50 μg/kg). (D) Rapamycin increased the level of transforming growth factor (TGF)-β. There were no significant differences between the 150, 250, and 500 μg/kg rapamycin-treated groups. *_P<_0.05.
Figure 10
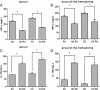
Levels of cytokines around the hematoma after rapamycin treatment. (A) Compared with the intracerebral hemorrhage (ICH) group, the levels of **interferon (IFN)-**γ were reduced in the rapamycin-treated groups except for the 50 μg/kg group. (B) Rapamycin-treated groups had higher levels of **interleukin (IL)**-10 than the ICH group, except for the 50 μg/kg group. (C) The ratio of IL-10 to IFN-γ was increased after treatment with rapamycin (150, 250, and 500 μg/kg, but not 50 μg/kg). (D) Rapamycin increased the level of transforming growth factor (TGF)-β. *_P<_0.05.
Figure 11
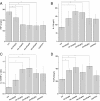
Effect of rapamycin on the levels of interferon (IFN)-γ and interleukin (IL)-10 at an autologous blood-injection model of intracerebral hemorrhage (ICH). (A, B) Similar to the collagenase-injection model, the levels of IFN-γ was downregulated after treatment with rapamycin both in serum and around the hematoma in an autologous blood-injection model. (C, D) Rapamycin upregulated IL-10 both in serum and around the hematoma. AU, Autologous blood-injection model of ICH, CO, Collagenase-injection model of ICH, RA, Rapamycin. *_P<_0.05.
Similar articles
- Role for Target of Rapamycin (mTOR) Signal Pathway in Regulating Neuronal Injury after Intracerebral Hemorrhage.
Wang JP, Zhang MY. Wang JP, et al. Cell Physiol Biochem. 2017;41(1):145-153. doi: 10.1159/000455983. Epub 2017 Jan 18. Cell Physiol Biochem. 2017. PMID: 28214828 - Rapamycin protects against neuronal death and improves neurological function with modulation of microglia after experimental intracerebral hemorrhage in rats.
Li D, Liu F, Yang T, Jin T, Zhang H, Luo X, Wang M. Li D, et al. Cell Mol Biol (Noisy-le-grand). 2016 Sep 30;62(11):67-75. Cell Mol Biol (Noisy-le-grand). 2016. PMID: 27755955 - Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage.
Rolland WB, Lekic T, Krafft PR, Hasegawa Y, Altay O, Hartman R, Ostrowski R, Manaenko A, Tang J, Zhang JH. Rolland WB, et al. Exp Neurol. 2013 Mar;241:45-55. doi: 10.1016/j.expneurol.2012.12.009. Epub 2012 Dec 21. Exp Neurol. 2013. PMID: 23261767 Free PMC article. - Mammalian target of rapamycin (mTOR) signaling pathway and traumatic brain injury: A novel insight into targeted therapy.
Movahedpour A, Vakili O, Khalifeh M, Mousavi P, Mahmoodzadeh A, Taheri-Anganeh M, Razmeh S, Shabaninejad Z, Yousefi F, Behrouj H, Ghasemi H, Khatami SH. Movahedpour A, et al. Cell Biochem Funct. 2022 Apr;40(3):232-247. doi: 10.1002/cbf.3692. Epub 2022 Mar 8. Cell Biochem Funct. 2022. PMID: 35258097 Review. - [Research progress on mTOR signaling pathway and regulatory T cell nutrition metabolic regulation mechanism].
Wu M, Wang F. Wu M, et al. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2024 Jan;40(1):69-73. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2024. PMID: 38246179 Review. Chinese.
Cited by
- Role of p75 neurotrophin receptor in neuronal autophagy in intracerebral hemorrhage in rats through the mTOR signaling pathway.
Wang L, Tian M, Hao Y. Wang L, et al. Cell Cycle. 2020 Feb;19(3):376-389. doi: 10.1080/15384101.2019.1711318. Epub 2020 Jan 10. Cell Cycle. 2020. PMID: 31924125 Free PMC article. Retracted. - TREM (Triggering Receptor Expressed on Myeloid Cells)-1 Inhibition Attenuates Neuroinflammation via PKC (Protein Kinase C) δ/CARD9 (Caspase Recruitment Domain Family Member 9) Signaling Pathway After Intracerebral Hemorrhage in Mice.
Lu Q, Liu R, Sherchan P, Ren R, He W, Fang Y, Huang Y, Shi H, Tang L, Yang S, Zhang JH, Tang J. Lu Q, et al. Stroke. 2021 Jun;52(6):2162-2173. doi: 10.1161/STROKEAHA.120.032736. Epub 2021 May 5. Stroke. 2021. PMID: 33947214 Free PMC article. - Modulating the Immune Response Towards a Neuroregenerative Peri-injury Milieu After Cerebral Hemorrhage.
Klebe D, McBride D, Flores JJ, Zhang JH, Tang J. Klebe D, et al. J Neuroimmune Pharmacol. 2015 Dec;10(4):576-86. doi: 10.1007/s11481-015-9613-1. Epub 2015 May 7. J Neuroimmune Pharmacol. 2015. PMID: 25946986 Free PMC article. Review. - NT3P75-2 gene-modified bone mesenchymal stem cells improve neurological function recovery in mouse TBI model.
Wu K, Huang D, Zhu C, Kasanga EA, Zhang Y, Yu E, Zhang H, Ni Z, Ye S, Zhang C, Hu J, Zhuge Q, Yang J. Wu K, et al. Stem Cell Res Ther. 2019 Oct 24;10(1):311. doi: 10.1186/s13287-019-1428-1. Stem Cell Res Ther. 2019. PMID: 31651375 Free PMC article. - Identification of hub genes and small-molecule compounds related to intracerebral hemorrhage with bioinformatics analysis.
Liu Z, Zhang R, Chen X, Yao P, Yan T, Liu W, Yao J, Sokhatskii A, Gareev I, Zhao S. Liu Z, et al. PeerJ. 2019 Oct 25;7:e7782. doi: 10.7717/peerj.7782. eCollection 2019. PeerJ. 2019. PMID: 31667013 Free PMC article.
References
- Ingall T. Stroke–incidence, mortality, morbidity and risk. J Insur Med. 2004;36:143–152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous