The free radical scavenger Trolox dampens neuronal hyperexcitability, reinstates synaptic plasticity, and improves hypoxia tolerance in a mouse model of Rett syndrome - PubMed (original) (raw)
The free radical scavenger Trolox dampens neuronal hyperexcitability, reinstates synaptic plasticity, and improves hypoxia tolerance in a mouse model of Rett syndrome
Oliwia A Janc et al. Front Cell Neurosci. 2014.
Abstract
Rett syndrome (RS) causes severe cognitive impairment, loss of speech, epilepsy, and breathing disturbances with intermittent hypoxia. Also mitochondria are affected; a subunit of respiratory complex III is dysregulated, the inner mitochondrial membrane is leaking protons, and brain ATP levels seem reduced. Our recent assessment of mitochondrial function in MeCP2 (methyl-CpG-binding protein 2)-deficient mouse (Mecp2 (-) (/y)) hippocampus confirmed early metabolic alterations, an increased oxidative burden, and a more vulnerable cellular redox balance. As these changes may contribute to the manifestation of symptoms and disease progression, we now evaluated whether free radical scavengers are capable of improving neuronal and mitochondrial function in RS. Acute hippocampal slices of adult mice were incubated with the vitamin E derivative Trolox for 3-5 h. In Mecp2 (-) (/y) slices this treatment dampened neuronal hyperexcitability, improved synaptic short-term plasticity, and fully restored synaptic long-term potentiation (LTP). Furthermore, Trolox specifically attenuated the increased hypoxia susceptibility of Mecp2 (-) (/y) slices. Also, the anticonvulsive effects of Trolox were assessed, but the severity of 4-aminopyridine provoked seizure-like discharges was not significantly affected. Adverse side effects of Trolox on mitochondria can be excluded, but clear indications for an improvement of mitochondrial function were not found. Since several ion-channels and neurotransmitter receptors are redox modulated, the mitochondrial alterations and the associated oxidative burden may contribute to the neuronal dysfunction in RS. We confirmed in Mecp2 (-) (/y) hippocampus that Trolox dampens neuronal hyperexcitability, reinstates synaptic plasticity, and improves the hypoxia tolerance. Therefore, radical scavengers are promising compounds for the treatment of neuronal dysfunction in RS and deserve further detailed evaluation.
Keywords: free radical scavenger; mitochondrial metabolism; neurodevelopmental disorder; oxidative stress; reactive oxygen species (ROS); redox signaling; synaptic dysfunction; vitamin E.
Figures
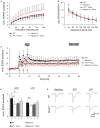
FIGURE 1
Trolox dampens neuronal hyperexcitability and reinstates LTP in _Mecp2_-/y hippocampus. (A) Input-output curves showing a significantly increased excitability in _Mecp2_-/y slices as compared to WT at all stimulation intensities (10–20 μA P < 0.05; 30–60 μA P < 0.01, 70–150 μA P < 0.001). Trolox (1 mM, 3–5h) abolished this genotypic difference. The plotted fEPSP amplitudes are normalized to the fiber volley of the respective slice. Displayed are the averages of 37–50 slices, and error bars represent standard deviations; for clarity they are shown for _Mecp2_-/y and _Mecp2_-/y plus Trolox only. (B) Paired-pulse facilitation (PPF), a measure of short-term plasticity, was less pronounced in _Mecp2_-/y than in WT slices for the shortest interpulse-interval tested. Trolox abolished this genotypic difference, but otherwise did not mediate any noticeable effects. Plotted are the averages of 35–52 slices; asterisks indicate statistically significant changes among WT and _Mecp2_-/y slices (*P < 0.05). (C) STP and LTP were less intense in _Mecp2_-/y slices. Trolox improved both types of plasticity in _Mecp2_-/y slices and LTP recovered to levels seen in untreated WT slices. In WT, Trolox dampened the extent of LTP to conditions typically found in untreated _Mecp2_-/y slices. Averages of 9–12 slices are shown. Error bars are included for every second data point of WT and _Mecp2_-/y slices only. LTP was induced by three consecutive trains of 100 Hz stimuli, lasting 1 s each (see arrow marks). (D) Comparison of the extent STP and LTP induced in the different groups. The number of slices analyzed is indicated at the bottom of the bars. Asterisks indicate statistically significant changes as compared to WT (**P < 0.01). (E) Sample traces of fEPSPs recorded for both genotypes in ACSF under baseline conditions, immediately after the 3rd high-frequency stimulation (STP), and 1 h after inducing potentiation (LTP). Stimulus artifacts are truncated.
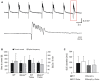
FIGURE 2
Trolox only shows a general tendency to dampen seizure susceptibility. (A) Seizure-like discharges (SLEs) recorded from an untreated _Mecp2_-/y slice in CA3 st. pyramidale. The lower trace shows a SLE (red box) at a stretched time scale. (B) Summary of the times to onset and the number of SLEs recorded in the two genotypes. Only in WT Trolox tended to postpone the onset of discharges as compared to untreated slices. The number of slices analyzed is indicated and applies to panel C as well. (C) Statistically significant differences in the duration of the individual SLEs among the genotypes were not observed. Trolox only tended to somewhat reduce the duration of the individual discharges in WT and _Mecp2_-/y slices.
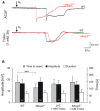
FIGURE 3
Trolox improves the hypoxia tolerance of _Mecp2_-/y hippocampus. (A) Extracellular DC potential deflections associated with HSD. Note that upon O2 withdrawal, HSD occurs earlier in _Mecp2_-/y than in WT slices. Incubation of slices with Trolox (1 mM, 3–5 h) selectively postponed HSD onset in _Mecp2_-/y slices to values typically observed in WT. The time point of O2 withdrawal is indicated by the arrows; oxygenation was restored 30 s upon HSD onset. Recordings were performed in CA1 st. radiatum. (B) Statistical comparison of the characteristic parameters of the HSD-associated DC potential shift, i.e., amplitude of the negative DC shift (plotted versus the left-hand ordinate in mV) as well as its time to onset and duration (both plotted versus the right-hand ordinate in s). Trolox postponed the onset of HSD in _Mecp2_-/y slices, but did not affect the other parameters or its properties in WT. The number of slices analyzed is reported; genotypic differences are indicated by asterisks (***P < 0.001).
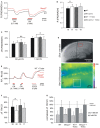
FIGURE 4
Trolox only slightly affects mitochondria. (A) Sample traces of the decreases in FAD/NADH ratio induced by CN- in untreated WT and _Mecp2_-/y slices. To clarify the baseline difference among genotypes, the traces were superimposed. Gray and red bars indicate the time points and duration of drug application to the WT and _Mecp2_-/y slice, respectively. (B) The FAD/NADH baseline ratio is moderately increased in _Mecp2_-/y slices, indicating intensified mitochondrial respiration. Trolox (1 mM, 3–5 h) did not affect basal respiration. Autofluorescence was analyzed in CA1 st. radiatum. Bar shading and patterns are identical for panels B, C, F (*P < 0.05). (C) Mitochondrial targeting by CN
- (3 min) arrests the respiratory chain, and thereby decreases the FAD/NADH ratio. Trolox slightly dampened these effects of CN- in _Mecp2_-/y slices but not in WT (** P < 0.01). (D) Rh123-labeled slice viewed under white-light illumination and 485 nm excitation. The overview image was taken with a 5x objective, and it also shows two of the vertical-oriented nylon strings immobilizing the slice. The red box indicates the zoomed field of view used for the Rh123 recordings. Fluorescence analyses were performed with a 40x objective and represent an integrated glial/neuronal signal of a tissue volume. Cell boundaries or mitochondrial structures are not identifiable, only the pyramidal cell layer is somewhat less intensely labeled. The white box indicates a typical region of interest analyzed (so st. oriens; sp st. pyramidale; sr st. radiatum). (E) Superimposed sample traces of the relative Rh123 fluorescence changes (F/Fo) evoked by CN- and FCCP in a Trolox-treated WT and _Mecp2_-/y slice. Drug treatment of the WT and _Mecp2_-/y slice is indicated by gray and red bars, respectively. (F) Uncoupling-mediated increases in Rh123 fluorescence indicating massive mitochondrial depolarization. Untreated _Mecp2_-/y slices showed less pronounced Rh123 increases upon FCCP treatment (5 μM, 5 min), suggesting a less negative Δψm Trolox dampened this genotypic difference. Data were analyzed in CA1 st. radiatum. (G) Summary of the CN--evoked increases in Rh123 fluorescence. Plotted data are normalized to the respective effects of FCCP, i.e., the maximum depolarization induced at the end of the experiment in each slice.
Similar articles
- Oxidative burden and mitochondrial dysfunction in a mouse model of Rett syndrome.
Grosser E, Hirt U, Janc OA, Menzfeld C, Fischer M, Kempkes B, Vogelgesang S, Manzke TU, Opitz L, Salinas-Riester G, Müller M. Grosser E, et al. Neurobiol Dis. 2012 Oct;48(1):102-14. doi: 10.1016/j.nbd.2012.06.007. Epub 2012 Jun 30. Neurobiol Dis. 2012. PMID: 22750529 - Systemic Radical Scavenger Treatment of a Mouse Model of Rett Syndrome: Merits and Limitations of the Vitamin E Derivative Trolox.
Janc OA, Hüser MA, Dietrich K, Kempkes B, Menzfeld C, Hülsmann S, Müller M. Janc OA, et al. Front Cell Neurosci. 2016 Nov 15;10:266. doi: 10.3389/fncel.2016.00266. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27895554 Free PMC article. - Neuronal Redox-Imbalance in Rett Syndrome Affects Mitochondria as Well as Cytosol, and Is Accompanied by Intensified Mitochondrial O 2 Consumption and ROS Release.
Can K, Menzfeld C, Rinne L, Rehling P, Kügler S, Golubiani G, Dudek J, Müller M. Can K, et al. Front Physiol. 2019 Apr 30;10:479. doi: 10.3389/fphys.2019.00479. eCollection 2019. Front Physiol. 2019. PMID: 31114506 Free PMC article. - Disturbed redox homeostasis and oxidative stress: Potential players in the developmental regression in Rett syndrome.
Müller M. Müller M. Neurosci Biobehav Rev. 2019 Mar;98:154-163. doi: 10.1016/j.neubiorev.2018.12.009. Epub 2019 Jan 9. Neurosci Biobehav Rev. 2019. PMID: 30639673 Review. - Exploring the possible link between MeCP2 and oxidative stress in Rett syndrome.
Filosa S, Pecorelli A, D'Esposito M, Valacchi G, Hajek J. Filosa S, et al. Free Radic Biol Med. 2015 Nov;88(Pt A):81-90. doi: 10.1016/j.freeradbiomed.2015.04.019. Epub 2015 May 8. Free Radic Biol Med. 2015. PMID: 25960047 Review.
Cited by
- Excitation and Inhibition Imbalance in Rett Syndrome.
Li W. Li W. Front Neurosci. 2022 Feb 18;16:825063. doi: 10.3389/fnins.2022.825063. eCollection 2022. Front Neurosci. 2022. PMID: 35250460 Free PMC article. Review. - Oral Feeding of an Antioxidant Cocktail as a Therapeutic Strategy in a Mouse Model of Rett Syndrome: Merits and Limitations of Long-Term Treatment.
Baroncelli L, Auel S, Rinne L, Schuster AK, Brand V, Kempkes B, Dietrich K, Müller M. Baroncelli L, et al. Antioxidants (Basel). 2022 Jul 20;11(7):1406. doi: 10.3390/antiox11071406. Antioxidants (Basel). 2022. PMID: 35883897 Free PMC article. - Dendrimer nanotherapy targeting of glial dysfunction improves inflammation and neurobehavioral phenotype in adult female Mecp2-heterozygous mouse model of Rett syndrome.
Khoury ES, Patel RV, O'Ferrall C, Fowler A, Sah N, Sharma A, Gupta S, Scafidi S, Kurtz JS, Olmstead SJ, Kudchadkar SR, Kannan RM, Blue ME, Kannan S. Khoury ES, et al. J Neurochem. 2024 May;168(5):841-854. doi: 10.1111/jnc.15960. Epub 2023 Sep 30. J Neurochem. 2024. PMID: 37777475 - Rescue of hyperexcitability in hippocampal CA1 neurons from Mecp2 (-/y) mouse through surface potential neutralization.
Balakrishnan S, Mironov SL. Balakrishnan S, et al. PLoS One. 2018 Apr 5;13(4):e0195094. doi: 10.1371/journal.pone.0195094. eCollection 2018. PLoS One. 2018. PMID: 29621262 Free PMC article. - Excitatory synapses are stronger in the hippocampus of Rett syndrome mice due to altered synaptic trafficking of AMPA-type glutamate receptors.
Li W, Xu X, Pozzo-Miller L. Li W, et al. Proc Natl Acad Sci U S A. 2016 Mar 15;113(11):E1575-84. doi: 10.1073/pnas.1517244113. Epub 2016 Feb 29. Proc Natl Acad Sci U S A. 2016. PMID: 26929363 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials