Clinical trials and late-stage drug development for Alzheimer's disease: an appraisal from 1984 to 2014 - PubMed (original) (raw)
Review
Clinical trials and late-stage drug development for Alzheimer's disease: an appraisal from 1984 to 2014
L S Schneider et al. J Intern Med. 2014 Mar.
Abstract
The modern era of drug development for Alzheimer's disease began with the proposal of the cholinergic hypothesis of memory impairment and the 1984 research criteria for Alzheimer's disease. Since then, despite the evaluation of numerous potential treatments in clinical trials, only four cholinesterase inhibitors and memantine have shown sufficient safety and efficacy to allow marketing approval at an international level. Although this is probably because the other drugs tested were ineffective, inadequate clinical development methods have also been blamed for the failures. Here, we review the development of treatments for Alzheimer's disease during the past 30 years, considering the drugs, potential targets, late-stage clinical trials, development methods, emerging use of biomarkers and evolution of regulatory considerations in order to summarize advances and anticipate future developments. We have considered late-stage Alzheimer's disease drug development from 1984 to 2013, including individual clinical trials, systematic and qualitative reviews, meta-analyses, methods, commentaries, position papers and guidelines. We then review the evolution of drugs in late clinical development, methods, biomarkers and regulatory issues. Although a range of small molecules and biological products against many targets have been investigated in clinical trials, the predominant drug targets have been the cholinergic system and the amyloid cascade. Trial methods have evolved incrementally: inclusion criteria have largely remained focused on mild-to-moderate Alzheimer's disease criteria, recently extending to early or prodromal Alzheimer disease or 'mild cognitive impairment due to Alzheimer's disease', for drugs considered to be disease modifying. The duration of trials has remained at 6-12 months for drugs intended to improve symptoms; 18- to 24-month trials have been established for drugs expected to attenuate clinical course. Cognitive performance, activities of daily living, global change and severity ratings have persisted as the primary clinically relevant outcomes. Regulatory guidance and oversight have evolved to allow for enrichment of early-stage Alzheimer's disease trial samples using biomarkers and phase-specific outcomes. In conclusion, validated drug targets for Alzheimer's disease remain to be developed. Only drugs that affect an aspect of cholinergic function have shown consistent, but modest, clinical effects in late-phase trials. There is opportunity for substantial improvements in drug discovery and clinical development methods.
Keywords: Alzheimer; clinical trial; dementia; drug development; treatment.
© 2014 The Association for the Publication of the Journal of Internal Medicine.
Conflict of interest statement
Disclosures and conflict of interest statements
LSS has received grants from the NIH P50 AG05142, R01 AG033288 and R01 AG037561, the State of California, the Alzheimer’s Association for a registry for dementia and cognitive impairment trials and grants or research support from Baxter, Genentech, Johnson & Johnson, Eli Lilly, Novartis and Pfizer. He has served as a consultant for and received consulting fees from Abbott Laboratories, AC Immune, Allon, AstraZeneca, Baxter, Biogen Idec, Biotie, Bristol-Myers Squibb, Elan, Eli Lilly, EnVivo, GlaxoSmithKline, Johnson & Johnson, Lundbeck, MedAvante, Merck, Novartis, Piramal, Pfizer, Roche, Sanofi, Servier, Takeda, Tau Rx, Toyama and Zinfandel.
PM has received research support from Lundbeck, Novartis and Pfizer and served as a consultant for and received consulting fees from Baxter, Bristol-Myers Squibb, Eli Lilly, Lundbeck, Merck, Merz, Novartis, Pfizer, Roche, Sanofi and Servier.
HF has received grants from CIHR, Pacific Alzheimers Research Foundation, NIH, the Alz Society of Canada. He has received research support or performed clinical trials with Biogen, Elan, GE Health, Lundbeck, Myriad, Glaxo, ONO, Bayer, Roche, Servier, Janssen, Eisai, Pfizer, Lilly, Sanofi, Novartis, Neotherapeutics, Boehringer Ingelheim. He has served as consultant for and received consulting fees from Elan, Lundbeck, Servier, Sanofi, Pfizer, Eisai, Eli Lilly, Novartis, Roche, Bayer, Neurocrine, ONO, Glaxo, Janssen, Boehringer Ingelheim. While on leave from UBC between 2009–2011, he was a paid full time employee of Bristol-Myers Squibb for which he received salary, stock, and stock options.
RJ has received research support from Eli Lilly, Janssen Alzheimer Immunotherapy, Servier, Abbott and Genentech and personal fees for advisory board meetings and/or chairmanship or lecture fees at symposia from Eisai, Elan, Eli Lilly, Janssen Alzheimer Immunotherapy, Lundbeck, Merz, Novartis, Nutricia, Otsuka, Pfizer and Roche.
VM and LP have no competing interests to disclose and no specific funding was received for writing this article. The views expressed in this article are the personal views of VM and LP and may not be understood or quoted as being made on behalf of or reflecting the position of the regulatory agency or any of the committees or working parties of the regulatory agencies for which the authors work.
BW has received research support from Dainippon Sumitomo Pharma Co. Ltd and has served as a consultant at advisory board meetings for AC Immune, Axon, Diagenic, Eli Lilly, Johnson & Johnson, Lundbeck, Merz, Novartis, Pfizer, Roche and Servier.
MK has received grants from the Alzheimer’s Association, Alzheimer’s Research and Prevention Foundation, Academy of Finland, EU 7th Framework and Swedish Research Council. She has served as a consultant at advisory board meetings for Elan, Johnson & Johnson, Merz, Novartis, Nutricia and Pfizer.
FM, NA, EG, and HF have no conflicts of interest to disclose.
Figures
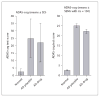
Fig. 1
Forest plots for 6-month trials of the three currently marketed cholinesterase inhibitors showing the general consistency of effect, mean drug–placebo differences on the ADAS-cog and 95% confidence intervals. The overall mean effect is 2.37 ADAS-cog points. Reproduced with permission from Birks [39].
Fig. 2
Outcomes of clinical trials of cholinesterase inhibitors for mild cognitive impairment showing a general lack of cognitive and global effects in trials from 2 to 4 years in duration (except for one 24-week trial). The data show the point estimates and 95% confidence intervals of the drug–placebo differences. The Z-cognitive is a composite of the neuropsychological test battery used in the rivastigmine trial. Reproduced with permission from Raschetti et al. [74].
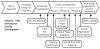
Fig. 3
The drug development process from preclinical development to approval. The focus of this review is phase 2 to phase 3 development. In general, the trials discussed were planned as phase 2, 2a or 3. Some phase 2 trials discussed were pivotal and could have been the basis for regulatory approval if the results had been positive. Modified from the FDA publication, Innovation or Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products.
Abbreviations: BLA: biologics license application; FDA: Food and Drug Administration; IND: Investigational new drug application; NDA: New drug application.
Fig. 4
Illustration of a randomized-start design for a regulatory clinical trial to demonstrate disease modification. Reproduced with permission from Leber [120].
Fig. 5
A comparison of the same effects of donepezil with standard deviation bars showing the distribution of drug and placebo outcomes and standard error bars indicating the precision of the outcomes measures. Reproduced with permission from Lindner et al. [2].
Similar articles
- Galantamine for Alzheimer's disease.
Olin J, Schneider L. Olin J, et al. Cochrane Database Syst Rev. 2001;(4):CD001747. doi: 10.1002/14651858.CD001747. Cochrane Database Syst Rev. 2001. PMID: 11687119 Updated. Review. - Galantamine for Alzheimer's disease.
Olin J, Schneider L. Olin J, et al. Cochrane Database Syst Rev. 2002;(3):CD001747. doi: 10.1002/14651858.CD001747. Cochrane Database Syst Rev. 2002. PMID: 12137632 Updated. Review. - Cholinesterase inhibitors for Alzheimer's disease.
Birks J. Birks J. Cochrane Database Syst Rev. 2006 Jan 25;2006(1):CD005593. doi: 10.1002/14651858.CD005593. Cochrane Database Syst Rev. 2006. PMID: 16437532 Free PMC article. Review. - Galantamine for Alzheimer's disease.
Olin J, Schneider L. Olin J, et al. Cochrane Database Syst Rev. 2001;(1):CD001747. doi: 10.1002/14651858.CD001747. Cochrane Database Syst Rev. 2001. PMID: 11279727 Updated. Review. - Clinical practice with anti-dementia drugs: A revised (third) consensus statement from the British Association for Psychopharmacology.
O'Brien JT, Holmes C, Jones M, Jones R, Livingston G, McKeith I, Mittler P, Passmore P, Ritchie C, Robinson L, Sampson EL, Taylor JP, Thomas A, Burns A. O'Brien JT, et al. J Psychopharmacol. 2017 Feb;31(2):147-168. doi: 10.1177/0269881116680924. Epub 2017 Jan 20. J Psychopharmacol. 2017. PMID: 28103749 Review.
Cited by
- The Dual Role of Amyloid Beta-Peptide in Oxidative Stress and Inflammation: Unveiling Their Connections in Alzheimer's Disease Etiopathology.
Fanlo-Ucar H, Picón-Pagès P, Herrera-Fernández V, Ill-Raga G, Muñoz FJ. Fanlo-Ucar H, et al. Antioxidants (Basel). 2024 Oct 8;13(10):1208. doi: 10.3390/antiox13101208. Antioxidants (Basel). 2024. PMID: 39456461 Free PMC article. Review. - The use of plasma exchange with albumin replacement in the management of Alzheimer's disease: a scoping review.
Cantero-Fortiz Y, Boada M. Cantero-Fortiz Y, et al. Front Neurol. 2024 Sep 30;15:1443132. doi: 10.3389/fneur.2024.1443132. eCollection 2024. Front Neurol. 2024. PMID: 39421573 Free PMC article. - IGF-1 and Glucocorticoid Receptors Are Potential Target Proteins for the NGF-Mimic Effect of _β_-Cyclocitral from Lavandula angustifolia Mill. in PC12 Cells.
An C, Gao L, Xiang L, Qi J. An C, et al. Int J Mol Sci. 2024 Sep 10;25(18):9763. doi: 10.3390/ijms25189763. Int J Mol Sci. 2024. PMID: 39337253 Free PMC article. - Recent advances in Alzheimer's disease: Mechanisms, clinical trials and new drug development strategies.
Zhang J, Zhang Y, Wang J, Xia Y, Zhang J, Chen L. Zhang J, et al. Signal Transduct Target Ther. 2024 Aug 23;9(1):211. doi: 10.1038/s41392-024-01911-3. Signal Transduct Target Ther. 2024. PMID: 39174535 Free PMC article. Review. - Alzheimer's disease: from early pathogenesis to novel therapeutic approaches.
Prajapati SK, Pathak A, Samaiya PK. Prajapati SK, et al. Metab Brain Dis. 2024 Aug;39(6):1231-1254. doi: 10.1007/s11011-024-01389-6. Epub 2024 Jul 24. Metab Brain Dis. 2024. PMID: 39046584 Review.
References
- Lindner MD, McArthur RA, Deadwyler S, Hampson R, Tariot PN. Development, Optimization and Use of Preclinical Behavioral Models to Maximize the Productivity of Drug Discovery for Alzheimer’s Disease. In: McArthur RA, Borsini F, editors. Animal and Translational Models for CNS Drug Discovery. San Diego: Academic Press; 2008. pp. 93–157.
- Pharmaceutical Research and Manufacturers of America. Researching Alzheimer’s Medicines: Setbacks and Stepping Stones. 2012.
- Giacobini E. Cholinesterases in human brain: the effect of cholinesterase inhibitors on Alzheimer’s disease and related disorders. In: Giacobini E, Pepeu G, editors. The Brain Cholinergic System in Health and Disease. Oxon, UK: Informa Healthcare; 2006. pp. 235–64.
- Schneider LS. Issues in Design and Conduct of Clinical Trials for Cognitive-Enhancing Drugs. In: McArthur RA, Borsini F, editors. Animal and Translational Models for CNS Drug Discovery. San Diego: Academic Press; 2008. pp. 21–76.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous