The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration - PubMed (original) (raw)
Review
The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration
Campbell D Lawson et al. Small GTPases. 2014.
Abstract
Rho GTPases play an essential role in regulating cell spreading, adhesion, and migration downstream of integrin engagement with the extracellular matrix. In this review, we focus on RhoA and Rac1--2 Rho GTPases that are required for efficient adhesion and migration--and describe how specific guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) regulate the extensive crosstalk that exists between them. In particular, we assess the role of GEFs and GAPs in light of recent, unexpected evidence concerning the spatiotemporal relationship between RhoA and Rac1 at the leading edge of migrating cells. Force is increasingly recognized as a key regulator of cell adhesion and we highlight the role of GEFs and GAPs in mechanotransduction, before debating the controversial role of tension in focal adhesion maturation.
Keywords: GAP; GEF; Rac1; Rho GTPase; RhoA; adhesion; integrin; mechanotransduction; migration.
Figures
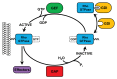
Figure 1. Regulation of Rho GTPase activity by GEFs, GAPs, and GDIs. At the membrane, inactive, GDP-bound Rho GTPases can be activated by GEFs, which catalyze the exchange of GDP for GTP. Once GTP-bound, Rho GTPases can bind to a variety of downstream effectors and elicit diverse responses. GAPs catalyze the GTPase-dependent hydrolysis of GTP back into GDP, thus inactivating Rho proteins. In the cytosol, Rho GTPases are bound by GDIs which prevent nucleotide exchange and bury the prenylated C-terminus, thus preventing degradation.
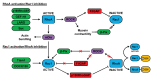
Figure 2. Crosstalk between RhoA and Rac1 in migrating cells. At the leading edge of migrating cells, RhoA can be activated by GEFs such as p190RhoGEF, GEF-H1, LARG, and Syx. At the cell rear, RhoA can restrict Rac1 activity via FilGAP and by negatively regulating the localization of β-Pix. RhoA-stimulated mDia activity may contribute to the subsequent increase in Rac1 activity at the leading edge, possibly by activating Src-dependent GEFs such as Tiam1 and DOCK180. Rac1 can inhibit RhoA via p190RhoGAP and the decrease in RhoA activity may further activate Rac1 by preventing FilGAP activation and by relieving the inhibition of β-Pix. The association of inactive RhoA with RhoGDI could also increase Rac1 activity as a result of the competitive binding of these 2 GTPases to GDI. See text for further details.
Similar articles
- Rho A and Rac1: Antagonists moving forward.
Salloum G, Jaafar L, El-Sibai M. Salloum G, et al. Tissue Cell. 2020 Aug;65:101364. doi: 10.1016/j.tice.2020.101364. Epub 2020 Apr 11. Tissue Cell. 2020. PMID: 32746999 Review. - Rho GTPases in collective cell migration.
Zegers MM, Friedl P. Zegers MM, et al. Small GTPases. 2014;5:e28997. doi: 10.4161/sgtp.28997. Epub 2014 May 9. Small GTPases. 2014. PMID: 25054920 Free PMC article. Review. - Therapeutic ultrasound bypasses canonical syndecan-4 signaling to activate rac1.
Mahoney CM, Morgan MR, Harrison A, Humphries MJ, Bass MD. Mahoney CM, et al. J Biol Chem. 2009 Mar 27;284(13):8898-909. doi: 10.1074/jbc.M804281200. Epub 2009 Jan 15. J Biol Chem. 2009. PMID: 19147498 Free PMC article. - The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho.
Leeuwen FN, Kain HE, Kammen RA, Michiels F, Kranenburg OW, Collard JG. Leeuwen FN, et al. J Cell Biol. 1997 Nov 3;139(3):797-807. doi: 10.1083/jcb.139.3.797. J Cell Biol. 1997. PMID: 9348295 Free PMC article. - Rho GEFs and GAPs: emerging integrators of extracellular matrix signaling.
Kutys ML, Yamada KM. Kutys ML, et al. Small GTPases. 2015;6(1):16-9. doi: 10.4161/21541248.2014.989792. Small GTPases. 2015. PMID: 25862162 Free PMC article.
Cited by
- Excitable Rho dynamics control cell shape and motility by sequentially activating ERM proteins and actomyosin contractility.
Marshall-Burghardt S, Migueles-Ramírez RA, Lin Q, El Baba N, Saada R, Umar M, Mavalwala K, Hayer A. Marshall-Burghardt S, et al. Sci Adv. 2024 Sep 6;10(36):eadn6858. doi: 10.1126/sciadv.adn6858. Epub 2024 Sep 6. Sci Adv. 2024. PMID: 39241071 Free PMC article. - Filling GAPs in our knowledge: ARHGAP11A and RACGAP1 act as oncogenes in basal-like breast cancers.
Lawson CD, Der CJ. Lawson CD, et al. Small GTPases. 2018 Jul 4;9(4):290-296. doi: 10.1080/21541248.2016.1220350. Epub 2016 Sep 26. Small GTPases. 2018. PMID: 27657701 Free PMC article. Review. - Bacterial genotoxins promote inside-out integrin β1 activation, formation of focal adhesion complexes and cell spreading.
Levi L, Toyooka T, Patarroyo M, Frisan T. Levi L, et al. PLoS One. 2015 Apr 13;10(4):e0124119. doi: 10.1371/journal.pone.0124119. eCollection 2015. PLoS One. 2015. PMID: 25874996 Free PMC article. - Thy-1 (CD90)-regulated cell adhesion and migration of mesenchymal cells: insights into adhesomes, mechanical forces, and signaling pathways.
Valdivia A, Avalos AM, Leyton L. Valdivia A, et al. Front Cell Dev Biol. 2023 Nov 30;11:1221306. doi: 10.3389/fcell.2023.1221306. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38099295 Free PMC article. Review. - The role of integrins in glaucoma.
Filla MS, Faralli JA, Peotter JL, Peters DM. Filla MS, et al. Exp Eye Res. 2017 May;158:124-136. doi: 10.1016/j.exer.2016.05.011. Epub 2016 May 13. Exp Eye Res. 2017. PMID: 27185161 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous