Fecal microbial composition of ulcerative colitis and Crohn's disease patients in remission and subsequent exacerbation - PubMed (original) (raw)
Fecal microbial composition of ulcerative colitis and Crohn's disease patients in remission and subsequent exacerbation
Edgar S Wills et al. PLoS One. 2014.
Abstract
Background: Limited studies have examined the intestinal microbiota composition in relation to changes in disease course of IBD over time. We aimed to study prospectively the fecal microbiota in IBD patients developing an exacerbation during follow-up.
Design: Fecal samples from 10 Crohn's disease (CD) and 9 ulcerative colitis (UC) patients during remission and subsequent exacerbation were included. Active disease was determined by colonoscopy and/or fecal calprotectine levels. Exclusion criteria were pregnancy, antibiotic use, enema use and/or medication changes between consecutive samples. The microbial composition was assessed by 16S rDNA pyrosequencing.
Results: After quality control, 6,194-11,030 sequences per sample were available for analysis. Patient-specific shifts in bacterial composition and diversity were observed during exacerbation compared to remission, but overarching shifts within UC or CD were not observed. Changes in the bacterial community composition between remission and exacerbation as assessed by Bray-Curtis dissimilarity, were significantly larger in CD versus UC patients (0.59 vs. 0.42, respectively; p = 0.025). Thiopurine use was found to be a significant cause of clustering as shown by Principal Coordinate Analysis and was associated with decreases in bacterial richness (Choa1 501.2 vs. 847.6 in non-users; p<0.001) and diversity (Shannon index: 5.13 vs. 6.78, respectively; p<0.01).
Conclusion: Shifts in microbial composition in IBD patients with changing disease activity over time seem to be patient-specific, and are more pronounced in CD than in UC patients. Furthermore, thiopurine use was found to be associated with the microbial composition and diversity, and should be considered when studying the intestinal microbiota in relation to disease course.
Conflict of interest statement
Competing Interests: MP has acted as a consultant for MSD and received payments for lectures from MSD, Falk, Pharma, Abvie and Ferring. The other authors have declared that no competing interests exist. The authors can confirm that the competing interests that they have declared do not alter their adherence to all PLOS ONE policies on sharing data and materials.
Figures
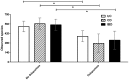
Figure 1. Relative abundance of bacterial phyla in fecal samples of nine UC (#1–9) and ten CD (#10–19) patients during remission and subsequent exacerbation.
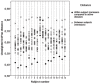
Figure 2. Mean number of observed species (OTUs) according to thiopurine use in Crohn’s disease (p = 2.57*10
−5 ), total Inflammatory Bowel Disease (p = 2.37*10 −4 ) and Ulcerative Colitis (p = 7.93*10 −4 ) patients. *: Significantly different (p<0.001).
Figure 3. Communities clustered using Principal Coordinates Analysis (PCoA) of the unweighted UniFrac distance matrix.
(A) PC1 and PC2 are plotted on _x_- and _y_-axes. Each point corresponds to a community colored according to thiopurine use. All samples are shown. The percentage of variation explained by the plotted principal coordinates is indicated on the axes. (B) PC1 and PC3 are plotted on x- and y-axis. Each point corresponds to a community colored according to disease location. Only samples from UC patients are included.
Figure 4. Within and between subjects pair-wise unweighted UniFrac distances for ulcerative colitis (#1–9) and Crohn’s disease (#10–19) patients.
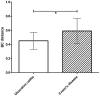
Figure 5. Mean within subjects pair-wise Bray-Curtis distances for ulcerative colitis and Crohn’s disease patients (p = 0.027).
*: Significantly different (p<0.05).
Similar articles
- Faecal Microbiota Dynamics and their Relation to Disease Course in Crohn's Disease.
Galazzo G, Tedjo DI, Wintjens DSJ, Savelkoul PHM, Masclee AAM, Bodelier AGL, Pierik MJ, Jonkers DMAE, Penders J. Galazzo G, et al. J Crohns Colitis. 2019 Sep 27;13(10):1273-1282. doi: 10.1093/ecco-jcc/jjz049. J Crohns Colitis. 2019. PMID: 30810207 Free PMC article. - Fecal microbial dysbiosis in Chinese patients with inflammatory bowel disease.
Ma HQ, Yu TT, Zhao XJ, Zhang Y, Zhang HJ. Ma HQ, et al. World J Gastroenterol. 2018 Apr 7;24(13):1464-1477. doi: 10.3748/wjg.v24.i13.1464. World J Gastroenterol. 2018. PMID: 29632427 Free PMC article. - Comparison of the fecal microbiota profiles between ulcerative colitis and Crohn's disease using terminal restriction fragment length polymorphism analysis.
Andoh A, Imaeda H, Aomatsu T, Inatomi O, Bamba S, Sasaki M, Saito Y, Tsujikawa T, Fujiyama Y. Andoh A, et al. J Gastroenterol. 2011 Apr;46(4):479-86. doi: 10.1007/s00535-010-0368-4. Epub 2011 Jan 21. J Gastroenterol. 2011. PMID: 21253779 - Role of antibiotics for treatment of inflammatory bowel disease.
Nitzan O, Elias M, Peretz A, Saliba W. Nitzan O, et al. World J Gastroenterol. 2016 Jan 21;22(3):1078-87. doi: 10.3748/wjg.v22.i3.1078. World J Gastroenterol. 2016. PMID: 26811648 Free PMC article. Review. - The association between the gut microbiota and the inflammatory bowel disease activity: a systematic review and meta-analysis.
Prosberg M, Bendtsen F, Vind I, Petersen AM, Gluud LL. Prosberg M, et al. Scand J Gastroenterol. 2016 Dec;51(12):1407-1415. doi: 10.1080/00365521.2016.1216587. Epub 2016 Aug 9. Scand J Gastroenterol. 2016. PMID: 27687331 Review.
Cited by
- Effects of Pretreatment with Bifidobacterium bifidum Using 16S Ribosomal RNA Gene Sequencing in a Mouse Model of Acute Colitis Induced by Dextran Sulfate Sodium.
Weng YJ, Jiang DX, Liang J, Ye SC, Tan WK, Yu CY, Zhou Y. Weng YJ, et al. Med Sci Monit. 2021 Mar 9;27:e928478. doi: 10.12659/MSM.928478. Med Sci Monit. 2021. PMID: 33686049 Free PMC article. - A Personalized Approach to Managing Inflammatory Bowel Disease.
Kingsley MJ, Abreu MT. Kingsley MJ, et al. Gastroenterol Hepatol (N Y). 2016 May;12(5):308-15. Gastroenterol Hepatol (N Y). 2016. PMID: 27499713 Free PMC article. - Current evidence and clinical relevance of drug-microbiota interactions in inflammatory bowel disease.
Becker HEF, Demers K, Derijks LJJ, Jonkers DMAE, Penders J. Becker HEF, et al. Front Microbiol. 2023 Feb 23;14:1107976. doi: 10.3389/fmicb.2023.1107976. eCollection 2023. Front Microbiol. 2023. PMID: 36910207 Free PMC article. Review. - Changes in duodenal tissue-associated microbiota following hookworm infection and consecutive gluten challenges in humans with coeliac disease.
Giacomin P, Zakrzewski M, Jenkins TP, Su X, Al-Hallaf R, Croese J, de Vries S, Grant A, Mitreva M, Loukas A, Krause L, Cantacessi C. Giacomin P, et al. Sci Rep. 2016 Nov 9;6:36797. doi: 10.1038/srep36797. Sci Rep. 2016. PMID: 27827438 Free PMC article. - The fecal microbiota as a biomarker for disease activity in Crohn's disease.
Tedjo DI, Smolinska A, Savelkoul PH, Masclee AA, van Schooten FJ, Pierik MJ, Penders J, Jonkers DM. Tedjo DI, et al. Sci Rep. 2016 Oct 13;6:35216. doi: 10.1038/srep35216. Sci Rep. 2016. PMID: 27734914 Free PMC article.
References
- Scharl M, Rogler G (2012) Inflammatory bowel disease pathogenesis: what is new? Curr Opin Gastroenterol 28: 301–309. - PubMed
- Blumenstein I, Bock H, Weber C, Rambow A, Tacke W, et al. (2008) Health care and cost of medication for inflammatory bowel disease in the Rhein-Main region, Germany: a multicenter, prospective, internet-based study. Inflamm Bowel Dis 14: 53–60. - PubMed
- Buchanan J, Wordsworth S, Ahmad T, Perrin A, Vermeire S, et al. (2011) Managing the long term care of inflammatory bowel disease patients: The cost to European health care providers. J Crohns Colitis 5: 301–316. - PubMed
- van der Valk ME, Mangen MJ, Leenders M, Dijkstra G, van Bodegraven AA, et al. (2014) Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFalpha therapy: results from the COIN study. Gut. - PubMed
- Talley NJ, Abreu MT, Achkar JP, Bernstein CN, Dubinsky MC, et al.. (2011) An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am J Gastroenterol 106 Suppl 1: S2–25; quiz S26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This study was financially supported by grants form the Academic Foundation of Maastricht University Medical Center+ and by the IBD Research Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical