Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma - PubMed (original) (raw)
Clinical Trial
doi: 10.1007/s00262-014-1528-9. Epub 2014 Mar 8.
Bianca Heemskerk, Harm van Tinteren, Rob R H van den Brom, Geke A P Hospers, Alfonsus J M van den Eertwegh, Ellen W Kapiteijn, Jan Willem B de Groot, Patricia Soetekouw, Rob L Jansen, Edward Fiets, Andrew J S Furness, Alexandra Renn, Marcin Krzystanek, Zoltan Szallasi, Paul Lorigan, Martin E Gore, Ton N M Schumacher, John B A G Haanen, James M G Larkin, Christian U Blank
Affiliations
- PMID: 24609989
- PMCID: PMC11029318
- DOI: 10.1007/s00262-014-1528-9
Clinical Trial
Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma
Sander Kelderman et al. Cancer Immunol Immunother. 2014 May.
Abstract
Introduction: Ipilimumab, a cytotoxic T lymphocyte-associated antigen-4 blocking antibody, has improved overall survival (OS) in metastatic melanoma in phase III trials. However, about 80 % of patients fail to respond, and no predictive markers for benefit from therapy have been identified. We analysed a 'real world' population of patients treated with ipilimumab to identify markers for treatment benefit.
Methods: Patients with advanced cutaneous melanoma were treated in the Netherlands (NL) and the United Kingdom (UK) with ipilimumab at 3 mg/kg. Baseline characteristics and peripheral blood parameters were assessed, and patients were monitored for the occurrence of adverse events and outcomes.
Results: A total of 166 patients were treated in the Netherlands. Best overall response and disease control rates were 17 and 35 %, respectively. Median follow-up was 17.9 months, with a median progression-free survival of 2.9 months. Median OS was 7.5 months, and OS at 1 year was 37.8 % and at 2 years was 22.9 %. In a multivariate model, baseline serum lactate dehydrogenase (LDH) was demonstrated to be the strongest predictive factor for OS. These findings were validated in an independent cohort of 64 patients from the UK.
Conclusion: In both the NL and UK cohorts, long-term benefit of ipilimumab treatment was unlikely for patients with baseline serum LDH greater than twice the upper limit of normal. In the absence of prospective data, clinicians treating melanoma may wish to consider the data presented here to guide patient selection for ipilimumab therapy.
Conflict of interest statement
G. A. P. Hospers, A. J. M. van den Eertwegh, E. W. Kapiteijn, J. W. de Groot, P. Lorigan, M. E. Gore, J. B. A. G. Haanen, J. M. G. Larkin and C. U. Blank have participated in advisory board meetings of Bristol-Myers Squibb for which the faculty has received compensation. P Lorigan has received support for travel and compensation for educational and speaker bureau activities. J. M. G. Larkin and M. E. Gore acknowledge National Health Service funding to the National Institute for Health Research Biomedical Research Centre at the Royal Marsden Hospital. J. B. A. G. Haanen and T. N. M. Schumacher are members of the Bristol-Meyers Squibb Immuno-Oncology network and have furthermore received a grant for translational research from Bristol-Meyers Squibb. C. U. Blank receives funding for an investigator-initiated study from Bristol-Myers Squibb. All other authors declared that they have no conflict of interest.
Figures
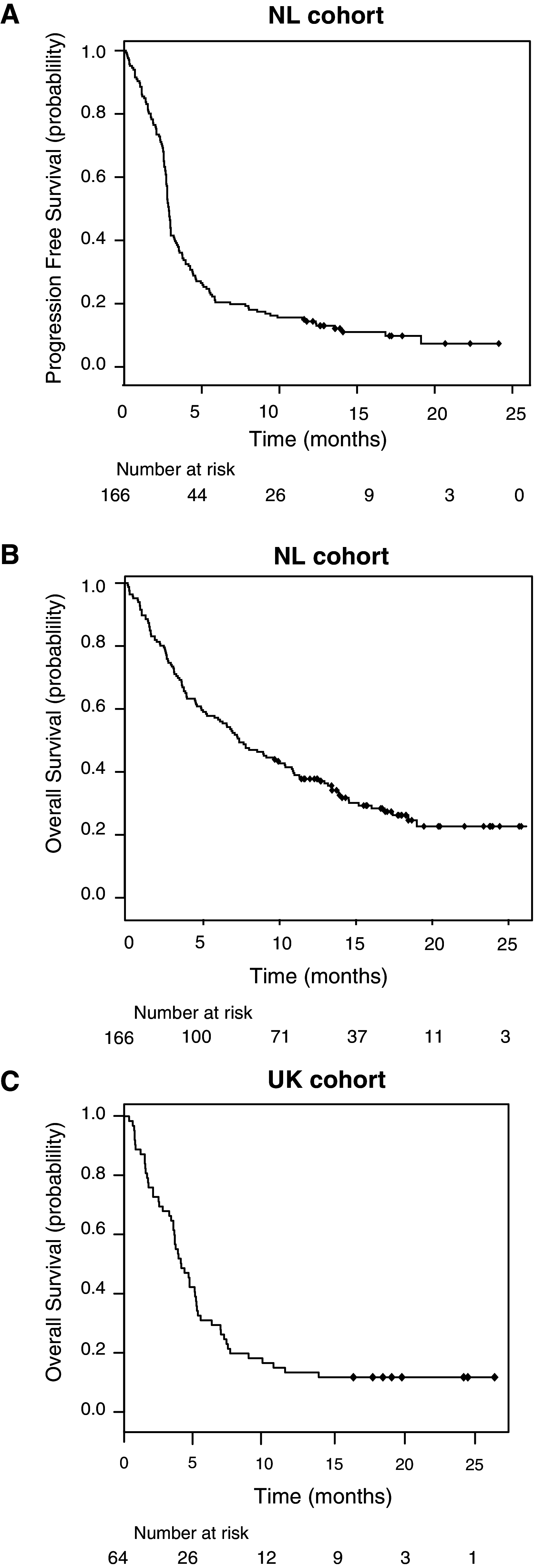
Fig. 1
Kaplan–Meier curves for progression-free survival and OS of NL and UK cohort. Progression-free survival curve for a the NL cohort and OS curves for b the NL and c UK patient cohorts are shown
Fig. 2
Kaplan–Meier curves for OS stratified by blood parameters. OS is shown when stratifying for blood values of a baseline absolute lymphocyte count (ALC), b ALC at week 6, c slope of ALC after two infusions, d baseline lactate dehydrogenase (LDH), e S100 and f baseline erythrocyte sedimentation rate (ESR). ULN upper limit of normal. P < 0.05 is statistically significant
Fig. 3
Patient selection based on baseline LDH for NL and UK cohort. Upper part of panel a shows the stratification of patients based on their upper limit of normal (ULN) baseline values of serum lactate dehydrogenase (LDH) in the NL cohort. For six patients, baseline LDH values were not available and these were excluded from further analyses. Lower part shows patients grouped by their response to therapy and the occurrence of toxicities. A statistically significant difference in OS was observed when stratifying by 1× ULN baseline LDH. However, seven patients with a clinical response upon ipilimumab treatment would have been missed when implementing this cut-off. Only two patients with a clinical response remained in the LDH-high subgroup when stratifying by 2× ULN of baseline LDH. Toxicities in the high and low LDH subgroups were similar. Panel b shows the stratification by baseline LDH values of patients from the UK cohort. At a cut-off of 1× ULN of baseline LDH, there is no significant influence on OS. Stratification by 2× ULN baseline LDH does significantly increase OS, and no patients in the LDH-high subgroup survive beyond the first year after treatment initiation. Percentages may not add up to 100 due to rounding. HR hazard ratio, CR complete response, PR partial response, SD stable disease, PD progressive disease, NE non-evaluable
Similar articles
- Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab.
Balatoni T, Ladányi A, Fröhlich G, Czirbesz K, Kovács P, Pánczél G, Bence E, Plótár V, Liszkay G. Balatoni T, et al. Pathol Oncol Res. 2020 Jan;26(1):317-325. doi: 10.1007/s12253-018-0466-9. Epub 2018 Sep 17. Pathol Oncol Res. 2020. PMID: 30225783 - Prognostic score for patients with advanced melanoma treated with ipilimumab.
Diem S, Kasenda B, Martin-Liberal J, Lee A, Chauhan D, Gore M, Larkin J. Diem S, et al. Eur J Cancer. 2015 Dec;51(18):2785-91. doi: 10.1016/j.ejca.2015.09.007. Epub 2015 Nov 18. Eur J Cancer. 2015. PMID: 26597444 - Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab.
Martens A, Wistuba-Hamprecht K, Geukes Foppen M, Yuan J, Postow MA, Wong P, Romano E, Khammari A, Dreno B, Capone M, Ascierto PA, Di Giacomo AM, Maio M, Schilling B, Sucker A, Schadendorf D, Hassel JC, Eigentler TK, Martus P, Wolchok JD, Blank C, Pawelec G, Garbe C, Weide B. Martens A, et al. Clin Cancer Res. 2016 Jun 15;22(12):2908-18. doi: 10.1158/1078-0432.CCR-15-2412. Epub 2016 Jan 19. Clin Cancer Res. 2016. PMID: 26787752 Free PMC article. - Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma.
Lipson EJ, Drake CG. Lipson EJ, et al. Clin Cancer Res. 2011 Nov 15;17(22):6958-62. doi: 10.1158/1078-0432.CCR-11-1595. Epub 2011 Sep 7. Clin Cancer Res. 2011. PMID: 21900389 Free PMC article. Review. - The ipilimumab lesson in melanoma: achieving long-term survival.
Delyon J, Maio M, Lebbé C. Delyon J, et al. Semin Oncol. 2015 Jun;42(3):387-401. doi: 10.1053/j.seminoncol.2015.02.005. Epub 2015 Feb 17. Semin Oncol. 2015. PMID: 25965357 Review.
Cited by
- The full blood count as a biomarker of outcome and toxicity in ipilimumab-treated cutaneous metastatic melanoma.
Khoja L, Atenafu EG, Templeton A, Qye Y, Chappell MA, Saibil S, Hogg D, Butler MO, Joshua AM. Khoja L, et al. Cancer Med. 2016 Oct;5(10):2792-2799. doi: 10.1002/cam4.878. Epub 2016 Sep 29. Cancer Med. 2016. PMID: 27683208 Free PMC article. - Regulation of Ovarian Cancer Prognosis by Immune Cells in the Tumor Microenvironment.
Drakes ML, Stiff PJ. Drakes ML, et al. Cancers (Basel). 2018 Sep 1;10(9):302. doi: 10.3390/cancers10090302. Cancers (Basel). 2018. PMID: 30200478 Free PMC article. Review. - Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab.
Balatoni T, Ladányi A, Fröhlich G, Czirbesz K, Kovács P, Pánczél G, Bence E, Plótár V, Liszkay G. Balatoni T, et al. Pathol Oncol Res. 2020 Jan;26(1):317-325. doi: 10.1007/s12253-018-0466-9. Epub 2018 Sep 17. Pathol Oncol Res. 2020. PMID: 30225783 - Inflammatory Cytokines and ctDNA Are Biomarkers for Progression in Advanced-Stage Melanoma Patients Receiving Checkpoint Inhibitors.
Pedersen JG, Madsen AT, Gammelgaard KR, Aggerholm-Pedersen N, Sørensen BS, Øllegaard TH, Jakobsen MR. Pedersen JG, et al. Cancers (Basel). 2020 May 30;12(6):1414. doi: 10.3390/cancers12061414. Cancers (Basel). 2020. PMID: 32486146 Free PMC article. - Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer.
Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, Ponce S, Ares LP, Leroy L, Audigier-Valette C, Felip E, Zerón-Medina J, Garrido P, Brosseau S, Zalcman G, Mazieres J, Caramela C, Lahmar J, Adam J, Chaput N, Soria JC, Besse B. Mezquita L, et al. JAMA Oncol. 2018 Mar 1;4(3):351-357. doi: 10.1001/jamaoncol.2017.4771. JAMA Oncol. 2018. PMID: 29327044 Free PMC article.
References
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, Ravetch JV, Allison JP, Quezada SA. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–1710. doi: 10.1084/jem.20130579. - DOI - PMC - PubMed
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. - DOI - PMC - PubMed
- Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. - PubMed
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. - DOI - PMC - PubMed
- Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis S, Lowy I, White DE, Rosenberg SA. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30(8):825–830. doi: 10.1097/CJI.0b013e318156e47e. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical