Essential role of hemoglobin beta-93-cysteine in posthypoxia facilitation of breathing in conscious mice - PubMed (original) (raw)
Essential role of hemoglobin beta-93-cysteine in posthypoxia facilitation of breathing in conscious mice
Benjamin Gaston et al. J Appl Physiol (1985). 2014.
Abstract
When erythrocyte hemoglobin (Hb) is fully saturated with O2, nitric oxide (NO) covalently binds to the cysteine 93 residue of the Hb β-chain (B93-CYS), forming S-nitrosohemoglobin. Binding of NO is allosterically coupled to the O2 saturation of Hb. As saturation falls, the NO group on B93-CYS is transferred to thiols in the erythrocyte, and in the plasma, forming circulating S-nitrosothiols. Here, we studied whether the changes in ventilation during and following exposure to a hypoxic challenge were dependent on erythrocytic B93-CYS. Studies were performed in conscious mice in which native murine Hb was replaced with human Hb (hB93-CYS mice) and in mice in which murine Hb was replaced with human Hb containing an alanine rather than cysteine at position 93 on the Bchain (hB93-ALA). Both strains expressed human γ-chain Hb, likely allowing a residual element of S-nitrosothiol-dependent signaling. While resting parameters and initial hypoxic (10% O2, 90% N2) ventilatory responses were similar in hB93-CYS mice and hB93-ALA mice, the excitatory ventilatory responses (short-term potentiation) that occurred once the mice were returned to room air were markedly diminished in hB93-ALA mice. Further, short-term potentiation responses were virtually absent in mice with bilateral transection of the carotid sinus nerves. These data demonstrate that hB93-CYS plays an essential role in mediating carotid sinus nerve-dependent short-term potentiation, an important mechanism for recovery from acute hypoxia.
Keywords: S-nitrosothiol; S-nitrosylation; hemoglobin; hypoxia; posthypoxia; ventilation.
Copyright © 2014 the American Physiological Society.
Figures
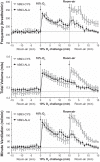
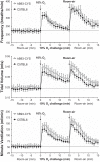
Fig. 1.
Frequency of breathing (top panel), tidal volume (middle panel), and minute ventilation (bottom panel) in conscious freely moving hB93-CYS mice (n = 15) and hB93-ALA mice (n = 16) before and during a hypoxic challenge (10% O2, 90% N2) and upon subsequent return to room air. The data are presented as means ± SE. Note that the horizontal dashed lines in the panels of Figs. 1–3 and Figs. 5 and 6 denote the average values recorded before hypoxic challenge (data taken from both groups of mice).
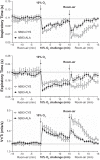
Fig. 2.
Inspiratory time (top panel), expiratory time (middle panel), and tidal volume/inspiratory time (V
t
/T
i
) (bottom panel) in conscious freely moving hB93-CYS mice (n = 15) and hB93-ALA mice (n = 16) before and during hypoxic challenge (10% O2, 90% N2) and upon return to room air. The data are presented as means ± SE.
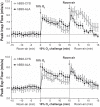
Fig. 3.
Peak inspiratory (Insp) flow (top panel) and peak expiratory (Exp) flow (bottom panel) in conscious freely moving hB93-CYS mice (n = 15) and hB93-ALA mice (n = 16) before and during hypoxic challenge (10% O2, 90% N2) and upon return to room air. The data are presented as means ± SE.
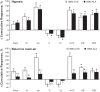
Fig. 4.
Total responses (cumulative %change from pre values) in ventilatory parameters in hB93-CYS mice (n = 15) and hB93-ALA mice (n = 16) during hypoxic challenge (top panel) and upon return to room air (bottom panel). The data are presented as means ± SE. Freq, breathing frequency; V
t
, tidal volume; V
m
, minute ventilation; T
i
, inspiratory time; T
e
, expiratory time; PIF, peak inspiratory flow; PEF, peak expiratory flow; *P < 0.05, significant response. †P < 0.05, hB93-ALA mice vs. hB93-CYS mice.
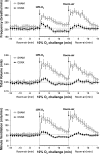
Fig. 5.
Frequency of breathing (top panel), tidal volume (middle panel), and minute ventilation (bottom panel) in conscious freely moving hB93-CYS mice (n = 15) and C57BL6 mice (n = 13) before and during hypoxic challenge (10% O2, 90% N2) and upon subsequent return to room air. The data are presented as means ± SE.
Fig. 6.
Frequency of breathing (top panel), tidal volume (middle panel), and minute ventilation (bottom panel) in conscious freely moving sham-operated C57BL6 (SHAM) mice (n = 9) or bilateral carotid sinus nerve-transected C57BL6 (CSNX) mice (n = 9) before and during hypoxic challenge (10% O2, 90% N2) and upon subsequent return to room air. The data are presented as means ± SE.
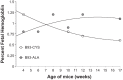
Fig. 7.
Percent fetal hemoglobin in B93-CYS and B93-ALA mice. Each point is from one mouse.
Similar articles
- Hemoglobin β93 Cysteine Is Not Required for Export of Nitric Oxide Bioactivity From the Red Blood Cell.
Sun CW, Yang J, Kleschyov AL, Zhuge Z, Carlström M, Pernow J, Wajih N, Isbell TS, Oh JY, Cabrales P, Tsai AG, Townes T, Kim-Shapiro DB, Patel RP, Lundberg JO. Sun CW, et al. Circulation. 2019 Jun 4;139(23):2654-2663. doi: 10.1161/CIRCULATIONAHA.118.039284. Epub 2019 Mar 25. Circulation. 2019. PMID: 30905171 Free PMC article. - Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation.
Allen BW, Stamler JS, Piantadosi CA. Allen BW, et al. Trends Mol Med. 2009 Oct;15(10):452-60. doi: 10.1016/j.molmed.2009.08.002. Epub 2009 Sep 24. Trends Mol Med. 2009. PMID: 19781996 Free PMC article. Review. - Essential Role of Hemoglobin βCys93 in Cardiovascular Physiology.
Premont RT, Stamler JS. Premont RT, et al. Physiology (Bethesda). 2020 Jul 1;35(4):234-243. doi: 10.1152/physiol.00040.2019. Physiology (Bethesda). 2020. PMID: 32490751 Free PMC article. Review. - SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation.
Isbell TS, Sun CW, Wu LC, Teng X, Vitturi DA, Branch BG, Kevil CG, Peng N, Wyss JM, Ambalavanan N, Schwiebert L, Ren J, Pawlik KM, Renfrow MB, Patel RP, Townes TM. Isbell TS, et al. Nat Med. 2008 Jul;14(7):773-7. doi: 10.1038/nm1771. Epub 2008 May 30. Nat Med. 2008. PMID: 18516054 Free PMC article. - Functional evidence that S-nitroso-L-cysteine may be a candidate carotid body neurotransmitter.
Getsy PM, Coffee GA, Bates JN, Baby SM, Seckler JM, Palmer LA, Lewis SJ. Getsy PM, et al. Neuropharmacology. 2025 Mar 1;265:110229. doi: 10.1016/j.neuropharm.2024.110229. Epub 2024 Nov 20. Neuropharmacology. 2025. PMID: 39577762
Cited by
- L-cysteine methyl ester overcomes the deleterious effects of morphine on ventilatory parameters and arterial blood-gas chemistry in unanesthetized rats.
Getsy PM, Baby SM, May WJ, Bates JN, Ellis CR, Feasel MG, Wilson CG, Lewis THJ, Gaston B, Hsieh YH, Lewis SJ. Getsy PM, et al. Front Pharmacol. 2022 Sep 28;13:968378. doi: 10.3389/fphar.2022.968378. eCollection 2022. Front Pharmacol. 2022. PMID: 36249760 Free PMC article. - Enhanced non-eupneic breathing following hypoxic, hypercapnic or hypoxic-hypercapnic gas challenges in conscious mice.
Getsy PM, Davis J, Coffee GA, May WJ, Palmer LA, Strohl KP, Lewis SJ. Getsy PM, et al. Respir Physiol Neurobiol. 2014 Dec 1;204:147-59. doi: 10.1016/j.resp.2014.09.006. Epub 2014 Sep 19. Respir Physiol Neurobiol. 2014. PMID: 25242462 Free PMC article. - The ventilatory depressant actions but not the antinociceptive effects of morphine are blunted in rats receiving intravenous infusion of L-cysteine ethyl ester.
Lewis THJ, May WJ, Young AP, Bates JN, Baby SM, Getsy PM, Ryan RM, Hsieh YH, Seckler JM, Lewis SJ. Lewis THJ, et al. Biomed Pharmacother. 2022 Dec;156:113939. doi: 10.1016/j.biopha.2022.113939. Epub 2022 Oct 28. Biomed Pharmacother. 2022. PMID: 36411626 Free PMC article. - L-cysteine ethyl ester prevents and reverses acquired physical dependence on morphine in male Sprague Dawley rats.
Bates JN, Getsy PM, Coffee GA, Baby SM, MacFarlane PM, Hsieh YH, Knauss ZT, Bubier JA, Mueller D, Lewis SJ. Bates JN, et al. Front Pharmacol. 2023 Dec 4;14:1303207. doi: 10.3389/fphar.2023.1303207. eCollection 2023. Front Pharmacol. 2023. PMID: 38111383 Free PMC article. - Loss of ganglioglomerular nerve input to the carotid body impacts the hypoxic ventilatory response in freely-moving rats.
Getsy PM, Coffee GA, Lewis SJ. Getsy PM, et al. Front Physiol. 2023 Mar 16;14:1007043. doi: 10.3389/fphys.2023.1007043. eCollection 2023. Front Physiol. 2023. PMID: 37008015 Free PMC article.
References
- Biscoe TJ, Pallot DJ. The carotid body chemoreceptor: an investigation in the mouse. Q J Exp Physiol 67: 557–576, 1982 - PubMed
- Chan NL, Kavanugh JS, Rogers PH, Arnone A. Crystallographic analysis of the interaction of nitric oxide with quaternary-T human hemoglobin. Biochemistry 43: 118–32, 2004 - PubMed
- Choi YB, Tenneti L, Le DA, Ortiz J, Bai G, Chen HSV, Lipton SA. Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat Neurosci 3: 15–21, 2000 - PubMed
- Do KQ, Benz B, Grima G, Gutteck-Amsler U, Kluge I, Salt TE. Nitric oxide precursor arginine and S-nitrosoglutathione in synaptic and glial function. Neurochem Int 29: 213–224, 1996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL101871/HL/NHLBI NIH HHS/United States
- U10 HL109250/HL/NHLBI NIH HHS/United States
- R01-HL-59337/HL/NHLBI NIH HHS/United States
- 1P-01-HL-101871/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials