Molecular mechanism of antibody-mediated activation of β-galactosidase - PubMed (original) (raw)
Molecular mechanism of antibody-mediated activation of β-galactosidase
Kutti R Vinothkumar et al. Structure. 2014.
Abstract
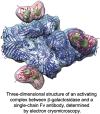
Binding of a single-chain Fv antibody to Escherichia coli β-galactosidase (β-gal) is known to stabilize the enzyme and activate several inactive point mutants, historically called antibody-mediated enzyme formation mutants. To understand the nature of this activation, we have determined by electron cryo-microscopy the structure of the complex between β-gal and the antibody scFv13R4. Our structure localizes the scFv13R4 binding site to the crevice between domains 1 and 3 in each β-gal subunit. The mutations that scFv13R4 counteracts are located between the antibody binding site and the active site of β-gal, at one end of the TIM-barrel that forms domain 3 where the substrate lactose is hydrolyzed. The mode of binding suggests how scFv stabilizes both the active site of β-gal and the tetrameric state.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Graphical abstract
Figure 1
Typical Regions of Raw Micrographs and Selected Images of Single Particles Field of view in typical micrographs of (A) the β-gal:Fv complex (image 19.59.55), (B) β-gal without antibody (image 01.49.47), and (C) Fv antibody alone (image 13.35.50). We also show a gallery of some selected particles of (D) β-gal alone and (E) complex. Because of the background of free scFv antibodies with 28 kDa MW, the images of the complex look noisy. Scale, 3,100 Å edge in (A–C) and 260 Å for each panel in (D) and (E).
Figure 2
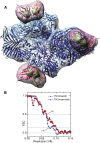
Surface-Contoured Structure of Complex between scFv13R4 and β-gal (A) Surface-contoured 3D map of β-gal alone (blue) and the difference map between β-gal and the β-gal:Fv complex (pink) with docked coordinates 1F4A of β-gal (Juers et al., 2000) and a typical Fv structure, taken from the 3A6B complex (Yokota et al., 2010) between hen egg lysozyme and a similar Fv. The maps were calculated using Frealign. The antibody domains are contoured at two different contour levels, showing the whole Fv domain at a lower contour level (pink) and the (just) resolved heavy and light chain densities at a higher contour level (green). (B) FSC plots for the β-gal:Fv complex. In blue squares, the FSC between experimental maps each calculated from half of the single-particle images shows a resolution at FSC 0.143 of ∼13 Å. In red circles, FSC between the 3D map and the density from the molecular model shows a resolution at FSC 0.5 of ∼15 Å. Figure S2 shows a similar 3D map and FSC plot for the structure computed using Relion.
Figure 3
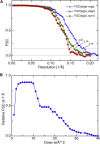
FSC Plots for the Structure of β-gal without Antibody (A) The FSC between two halves of the data for the structure of β-gal without antibody is shown for the simple sum of image frames (red diamonds), for the summed frames after alignment (green squares), and for the resolution-dependent weighted sums (blue triangles), showing resolutions of 6.8, 6.3, and 5.4 Å, respectively. (B) The weighting factor proportional to the FSC at ∼7 Å resolution is shown for 40 3D maps made from the first 40 single frames, each with a dose of 1 el/Å2. Figure S3 shows two additional plots: a tilt pair parameter plot to validate the overall procedure and high-resolution noise substitution to validate resolution.
Figure 4
Geometrical Relationship between the Fv Antibody and Key Residues in the Enzyme Each Fv antibody binds to an extended surface of one β-gal subunit that is made up of part of the N-terminal domain 1 and part of domain 3, the core β-barrel catalytic domain that lies at the heart of each of the β-gal subunits. The illustration shows the entire structure (top left); one β-gal subunit with each of the five domains in different colors (top right); and parts of domains 1 and 3 from one β-gal subunit (bottom right and bottom left), rotated ∼30° to the left, showing the region that is in contact with the (red) Fv. The amino acid E358K (yellow) that is mutated in AMEF959, the amino acid G207D (yellow) that is mutated in AMEF645, and the two active-site residues E461 and H540 are labeled. A purple atomic model of IPTG (from PDB
3DYO
) shows the location of the substrate binding site. The most extensive contacts, shown at bottom left and listed in Table 1, are between the Fv heavy chain and domain 3 of β-gal, with the antibody surface shown in dark gray and the β-gal surface in cyan. Figure S4 shows a stereo plot of the same region.
Similar articles
- Structure-function relationships in Gan42B, an intracellular GH42 β-galactosidase from Geobacillus stearothermophilus.
Solomon HV, Tabachnikov O, Lansky S, Salama R, Feinberg H, Shoham Y, Shoham G. Solomon HV, et al. Acta Crystallogr D Biol Crystallogr. 2015 Dec 1;71(Pt 12):2433-48. doi: 10.1107/S1399004715018672. Epub 2015 Nov 26. Acta Crystallogr D Biol Crystallogr. 2015. PMID: 26627651 - Superactive β-galactosidase inclusion bodies.
Flores SS, Nolan V, Perillo MA, Sánchez JM. Flores SS, et al. Colloids Surf B Biointerfaces. 2019 Jan 1;173:769-775. doi: 10.1016/j.colsurfb.2018.10.049. Epub 2018 Oct 22. Colloids Surf B Biointerfaces. 2019. PMID: 30384274 - The structure of E. coli beta-galactosidase.
Matthews BW. Matthews BW. C R Biol. 2005 Jun;328(6):549-56. doi: 10.1016/j.crvi.2005.03.006. C R Biol. 2005. PMID: 15950161 Review. - ATP synthase from Escherichia coli: Mechanism of rotational catalysis, and inhibition with the ε subunit and phytopolyphenols.
Nakanishi-Matsui M, Sekiya M, Futai M. Nakanishi-Matsui M, et al. Biochim Biophys Acta. 2016 Feb;1857(2):129-140. doi: 10.1016/j.bbabio.2015.11.005. Epub 2015 Nov 14. Biochim Biophys Acta. 2016. PMID: 26589785 Review.
Cited by
- Antibody-mediated enzyme formation: Its legacy at age fifty-four.
Strom R, Celada F. Strom R, et al. J Mol Recognit. 2021 Dec;34(12):e2931. doi: 10.1002/jmr.2931. Epub 2021 Oct 24. J Mol Recognit. 2021. PMID: 34693572 Free PMC article. Review. - The potential use of single-particle electron microscopy as a tool for structure-based inhibitor design.
Rawson S, McPhillie MJ, Johnson RM, Fishwick CWG, Muench SP. Rawson S, et al. Acta Crystallogr D Struct Biol. 2017 Jun 1;73(Pt 6):534-540. doi: 10.1107/S2059798317004077. Epub 2017 Apr 20. Acta Crystallogr D Struct Biol. 2017. PMID: 28580915 Free PMC article. - Measuring the effects of particle orientation to improve the efficiency of electron cryomicroscopy.
Naydenova K, Russo CJ. Naydenova K, et al. Nat Commun. 2017 Sep 20;8(1):629. doi: 10.1038/s41467-017-00782-3. Nat Commun. 2017. PMID: 28931821 Free PMC article. - Comparison of optimal performance at 300keV of three direct electron detectors for use in low dose electron microscopy.
McMullan G, Faruqi AR, Clare D, Henderson R. McMullan G, et al. Ultramicroscopy. 2014 Dec;147:156-63. doi: 10.1016/j.ultramic.2014.08.002. Epub 2014 Aug 9. Ultramicroscopy. 2014. PMID: 25194828 Free PMC article. - High-resolution cryo-EM proteasome structures in drug development.
Morris EP, da Fonseca PCA. Morris EP, et al. Acta Crystallogr D Struct Biol. 2017 Jun 1;73(Pt 6):522-533. doi: 10.1107/S2059798317007021. Epub 2017 May 31. Acta Crystallogr D Struct Biol. 2017. PMID: 28580914 Free PMC article.
References
- Bellare J.R., Davis H.T., Scriven L.E., Talmon Y. Controlled environment vitrification system: an improved sample preparation technique. J. Electron Microsc. Tech. 1988;10:87–111. - PubMed
- Celada F., Strom R. Antibody-induced conformational changes in proteins. Q. Rev. Biophys. 1972;5:395–425. - PubMed
- Crowther R.A., Henderson R., Smith J.M. MRC image processing programs. J. Struct. Biol. 1996;116:9–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases