ZiBuPiYin recipe protects db/db mice from diabetes-associated cognitive decline through improving multiple pathological changes - PubMed (original) (raw)
ZiBuPiYin recipe protects db/db mice from diabetes-associated cognitive decline through improving multiple pathological changes
Jing Chen et al. PLoS One. 2014.
Abstract
Multiple organ systems, including the brain, which undergoes changes that may increase the risk of cognitive decline, are adversely affected by diabetes mellitus (DM). Here, we demonstrate that type 2 diabetes mellitus (T2DM) db/db mice exhibited hippocampus-dependent memory impairment, which might associate with a reduction in dendritic spine density in the pyramidal neurons of brain, Aβ1-42 deposition in the prefrontal cortex (PFC) and hippocampus, and a decreased expression of neurostructural proteins including microtubule-associated protein (MAP2), a marker of dendrites, and postsynaptic density 95 (PSD95), a marker of excitatory synapses. To investigate the effects of the ZiBuPiYin recipe (ZBPYR), a traditional Chinese medicine recipe, on diabetes-related cognitive decline (DACD), db/db mice received daily administration of ZBPYR over an experimental period of 6 weeks. We then confirmed that ZBPYR rescued learning and memory performance impairments, reversed dendritic spine loss, reduced Aβ1-42 deposition and restored the expression levels of MAP2 and PSD95. The present study also revealed that ZBPYR strengthened brain leptin and insulin signaling and inhibited GSK3β overactivity, which may be the potential mechanism or underlying targets of ZBPYR. These findings conclude that ZBPYR prevents DACD, most likely by improving dendritic spine density and attenuating brain leptin and insulin signaling pathway injury. Our findings provide further evidence for the effects of ZBPYR on DACD.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
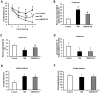
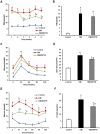
Figure 1. Effects of ZBPYR on the Morris water maze test in db/db mice.
(A) Learning performance of the animals was analyzed in the training trials by escape latency. DM/ZBPYR mice had shorter escape latency on the 4th and 5th day of training. (B–D) Memory retrieval performance was investigated in the probe test as the time required to search for the original platform location (B), time in the target quadrant where the platform had been located during training trials (C), and the number of crossing over the original platform location (D). (E–F) Performance in the visible platform version of the Morris water maze, which is not hippocampus-dependent. Escape latency (E) and swimming distance (F) were analyzed. Values are means ± S.D. from 17 mice in each group. *p<0.05 compared to control; # p<0.05 compared to DM.
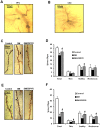
Figure 2. Effects of ZBPYR on dendritic spines in different regions of brain.
(A–B) Representative examples of a PFC neuron (A) and a CA1 neuron (B) visualized with Golgi staining. Scale bar = 100 µm. (C) Representative images of Golgi-stained PFC neurons from the different groups. Long arrow indicates a thin spine, short arrow indicates a mushroom spine and triangle indicates a stubby spine. Scale bar = 20 µm. (D) ZBPYR significantly increased total dendritic spine density and stubby spine quantity over a dendritic segment length of 50 µm. (E) Representative images of Golgi-stained hippocampal CA1 neurons from the different groups are shown. Long arrow indicates a thin spine, short arrow indicates a mushroom spine and triangle indicates a stubby spine. Scale bar = 20 µm. (F) ZBPYR increased total dendritic spine density and all three types spines quantity over a dendritic segment length of 50 µm. Values are means ± S.D. from 3 mice in each group. *p<0.05 compared to control; # p<0.05 compared to DM.
Figure 3. Effects of ZBPYR on Aβ1-42 deposition and the expression of neurostructural proteins in different regions of brain.
(A) Aβ1-42 deposition in the PFC, hippocampal CA1 and DG were visualized using immunohistochemistry. (B) The expression of MAP2 in the PFC and hippocampal CA1 was visualized using immunohistochemistry. (C) The expression of PSD95 in the hippocampal CA1 was visualized using immunohistochemistry. (D–F) The average IOD of the immunoreactive area for Aβ1-42, MAP2 and PSD95, are presented as bar graphs. ZBPYR significantly reduced Aβ1-42 deposition in the PFC and hippocampal CA1 and DG (D). ZBPYR significantly increased the expression of MAP2 in the PFC and hippocampal CA1 (E). ZBPYR significantly increased the expression of PSD95 in the hippocampal CA1 (F). Scale bar = 100 µm. Values are means ± S.D. from 3 mice in each group. *p<0.05 compared to control; # p<0.05 compared to DM.
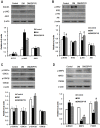
Figure 4. Effects of ZBPYR on brain leptin and insulin signaling. GSK3 activity and neurostructural protein expression in the hippocampus.
(A) ZBPYR did not alter SOCS3 expression, but significantly decreased p-JAK2 (Tyr1007/1008) expression. (B) ZBPYR reduced IRS2 that is phosphorylated at serine 731 and increased Akt phosphorylated at serine 473. (C) ZBPYR significantly impaired GSK3β activity by increasing p-Ser9GSK3β expression. (D) ZBPYR significantly increased MAP2 and PSD95 expression, and reduced MAP2 that is phosphorylated on tyrosine 1620/1623. Representative Western blots and bar graphs of gray-scale analysis are shown. β-actin was used as a loading control. Values are means ± S.D. from 4 mice in each group. *p<0.05 compared to control; # p<0.05 compared to DM.
Figure 5. Effects of ZBPYR on brain leptin and insulin signaling. GSK3 activity and neurostructural protein expression in the cerebral cortex.
(A) ZBPYR did not alter SOCS3 expression, but significantly decreased p-JAK2 (Tyr1007/1008) expression. (B) ZBPYR reduced p-IRS2 at serine 731 and increased p-Akt at serine 473. (C) ZBPYR significantly impaired GSK3β activity by increasing p-Ser9GSK3β expression. (D) ZBPYR significantly increased MAP2 and PSD95 expression, and reduced MAP2 that is phosphorylated on tyrosine 1620/1623. Representative Western blots and bar graphs of gray-scale analysis are shown. β-actin was used as a loading control. Values are means ± S.D. from 4 mice in each group. *p<0.05 compared to control; # p<0.05 compared to DM.
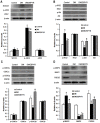
Figure 6. The anti-diabetic effects of ZBPYR on db/db mice.
(A) RBG was examined weekly during ZBPYR administration. ZBPYR significantly reduced glucose levels from the 3rd week to the 6th week. (B) FSI was accessed at the end of the administration period and after the animals had been starved for 12 h. (C–D) OGTT (2 g/kg body weight) was performed at the end of the administration period and after the animals had been starved for 14 h. Actual glucose levels were measured at the indicated times (C), and then the AUC was analyzed (D). (E–F) ITT (0.75 U/kg body weight) was performed at the end of the administration period and after the animals had been starved for 6 h. Actual glucose levels were measured at the indicated times (E), and then the AUC was analyzed (F). Values are means ± S.D. from 17 mice in each group. *p<0.05 compared to control; # p<0.05 compared to DM.
Similar articles
- The Alteration of ZiBuPiYin Recipe on Proteomic Profiling of Forebrain Postsynaptic Density of db/db Mice with Diabetes-Associated Cognitive Decline.
Chen J, Zhan L, Lu X, Xiao C, Sun N. Chen J, et al. J Alzheimers Dis. 2017;56(2):471-489. doi: 10.3233/JAD-160691. J Alzheimers Dis. 2017. PMID: 27886008 - ZiBu PiYin recipe prevents diabetes-associated cognitive decline in rats: possible involvement of ameliorating mitochondrial dysfunction, insulin resistance pathway and histopathological changes.
Sun Z, Zhan L, Liang L, Sui H, Zheng L, Sun X, Xie W. Sun Z, et al. BMC Complement Altern Med. 2016 Jul 8;16:200. doi: 10.1186/s12906-016-1177-y. BMC Complement Altern Med. 2016. PMID: 27393392 Free PMC article. - Neuroprotective Effects of ZiBuPiYin Recipe on db/db Mice via PI3K-Akt Signaling Pathway by Activating Grb2.
Ren WM, Weng ZB, Li X, Zhan LB. Ren WM, et al. Neural Plast. 2021 Jan 30;2021:8825698. doi: 10.1155/2021/8825698. eCollection 2021. Neural Plast. 2021. PMID: 33603781 Free PMC article. - ZiBuPiYin recipe ameliorates diabetes-associated cognitive decline by improving neuronal mitochondrial function in chronic psychologically stressed zucker diabetic fatty rats.
Sui H, Zhu L, Zhan L, Bi T, Zhang B. Sui H, et al. J Ethnopharmacol. 2023 Feb 10;302(Pt B):115947. doi: 10.1016/j.jep.2022.115947. Epub 2022 Nov 17. J Ethnopharmacol. 2023. PMID: 36403740 - Molecular mechanisms underlying hyperglycemia associated cognitive decline.
Gupta M, Pandey S, Rumman M, Singh B, Mahdi AA. Gupta M, et al. IBRO Neurosci Rep. 2022 Dec 13;14:57-63. doi: 10.1016/j.ibneur.2022.12.006. eCollection 2023 Jun. IBRO Neurosci Rep. 2022. PMID: 36590246 Free PMC article. Review.
Cited by
- Elevated glucose and oligomeric β-amyloid disrupt synapses via a common pathway of aberrant protein S-nitrosylation.
Akhtar MW, Sanz-Blasco S, Dolatabadi N, Parker J, Chon K, Lee MS, Soussou W, McKercher SR, Ambasudhan R, Nakamura T, Lipton SA. Akhtar MW, et al. Nat Commun. 2016 Jan 8;7:10242. doi: 10.1038/ncomms10242. Nat Commun. 2016. PMID: 26743041 Free PMC article. - Brain signaling systems in the Type 2 diabetes and metabolic syndrome: promising target to treat and prevent these diseases.
Shpakov AO, Derkach KV, Berstein LM. Shpakov AO, et al. Future Sci OA. 2015 Nov 1;1(3):FSO25. doi: 10.4155/fso.15.23. eCollection 2015 Nov. Future Sci OA. 2015. PMID: 28031898 Free PMC article. Review. - Obesity, diabetes, and leptin resistance promote tau pathology in a mouse model of disease.
Platt TL, Beckett TL, Kohler K, Niedowicz DM, Murphy MP. Platt TL, et al. Neuroscience. 2016 Feb 19;315:162-74. doi: 10.1016/j.neuroscience.2015.12.011. Epub 2015 Dec 14. Neuroscience. 2016. PMID: 26701291 Free PMC article. - The ZiBuPiYin recipe regulates proteomic alterations in brain mitochondria-associated ER membranes caused by chronic psychological stress exposure: Implications for cognitive decline in Zucker diabetic fatty rats.
Xu H, Zhou W, Zhan L, Sui H, Zhang L, Zhao C, Lu X. Xu H, et al. Aging (Albany NY). 2020 Nov 18;12(23):23698-23726. doi: 10.18632/aging.103894. Epub 2020 Nov 18. Aging (Albany NY). 2020. PMID: 33221746 Free PMC article. - Integrative Analyses of Genes Associated with Fulminant Type 1 Diabetes.
Ye X, Zeng T, Kong W, Chen LL. Ye X, et al. J Immunol Res. 2020 Oct 6;2020:1025857. doi: 10.1155/2020/1025857. eCollection 2020. J Immunol Res. 2020. PMID: 33083497 Free PMC article.
References
- Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, et al. (2010) Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology 75: 1195–1202. - PubMed
- Profenno LA, Porsteinsson AP, Faraone SV (2010) Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biological Psychiatry 67: 505–512. - PubMed
- Kopf D, Frölich L (2009) Risk of incident Alzheimer's disease in diabetic patients: a systematic review of prospective trials. Journal of Alzheimer's Disease 16: 677–685. - PubMed
- Zhao Q, Matsumoto K, Tsuneyama K, Tanaka K, Li F, et al. (2011) Diabetes-Induced Central Cholinergic Neuronal Loss and Cognitive Deficit Are Attenuated by Tacrine and a Chinese Herbal Prescription Kangen-Karyu: Elucidation in Type 2 Diabetes db/db Mice. J Pharmacol Sci 117: 230–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was financially supported by The Key Project of National Natural Science Foundation, China (No. 81230084), The Specialized Research Fund for the Doctoral Program of Higher Education, China (No. 20112105110006) and The Program for Professor of Special Appointment in Liaoning Province. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous