CTCF: an architectural protein bridging genome topology and function - PubMed (original) (raw)
Review
CTCF: an architectural protein bridging genome topology and function
Chin-Tong Ong et al. Nat Rev Genet. 2014 Apr.
Abstract
The eukaryotic genome is organized in the three-dimensional nuclear space in a specific manner that is both a cause and a consequence of its function. This organization is partly established by a special class of architectural proteins, of which CCCTC-binding factor (CTCF) is the best characterized. Although CTCF has been assigned various roles that are often contradictory, new results now help to draw a unifying model to explain the many functions of this protein. CTCF creates boundaries between topologically associating domains in chromosomes and, within these domains, facilitates interactions between transcription regulatory sequences. Thus, CTCF links the architecture of the genome to its function.
Figures
Figure 1. Features of CTCF binding sites in the genome
CTCF binding sites are associated with different genetic elements. The majority of sites are intergenic and co-localize with cohesin. In addition, a fraction of CTCF binding sites are located near RNA polymerase III (RNAPIII) type II genes (e.g. tRNA and SINE elements) and ETC loci, suggesting that TFIIIC and CTCF may cooperate in some aspects of the function of this protein. The 12 bp consensus sequence of CTCF sites is embedded within binding modules 2 and 3 as determined by the ChIP-exo technique. DNA methylation (filled red ball) of cytosine residues occurs at positions 2 and 12 of the consensus sequence in a subset of CTCF sites.
Figure 2. Regulation of CTCF binding to DNA
Constitutive CTCF sites present in cells from different tissues are present in non-methylated and nucleosome-free regions. Cell-type specific CTCF binding is partly regulated by differential DNA methylation and nucleosome occupancy across different cell-types. This suggests that cells may use ATP-dependent chromatin remodeling complexes to regulate nucleosome occupancy at specific CTCF sites and control the interaction of this protein with DNA. In addition, the methylation status of cell-type-specific CTCF binding sites may be determined by a combination of activities of de novo methyltransferases and TET enzymes that regulate the presence and levels of 5mC at specific sites. Immortalized cancer cell lines contain high levels of 5mC at CTCF sites, which correlates with the low CTCF occupancy in these cells (Filled red circle: methylated DNA; open circle: unmethylated DNA).
Figure 3. CTCF regulates enhancer-promoter interactions in a multi-gene cluster
The human _Pcdh_α gene cluster contains 13 similar, tandemly arranged, variable first exons (1 to 13, shown in blue if they are transcribed or in white if they are not) and two related c-type ubiquitous first exons (c1 and c2, shown in yellow). Each of these 15 variable first exons is adjacent to its own promoter and is spliced to three downstream constant exons (1 to 3, shown in black). _Pcdh_α alternate isoforms are expressed stochastically, whereas all the c-type isoforms are expressed ubiquitously in all cells. The SK-N-SH cells depicted here express isoforms 4, 8 and 12. Promoter choice and the formation of an active chromatin hub is mediated by CTCF-cohesin DNA looping between the distal HS5-1 enhancer and distinct promoters at the _Pcdh_α gene cluster. Individual variable exons (blue and white rectangles) or ubiquitous exons (yellow rectangles) may be expressed and joined to the three exons from the constant region (black rectangles) by pre-mRNA splicing. Binding of CTCF to the promoter preceding individual exons is correlated with the level of gene activity. The active promoters are distinguished from the inactive promoters by an enrichment for H3K4me3 and a depletion of DNA methylation, which leads to expression of the downstream genes (blue rectangles).
Figure 4. CTCF facilitates endodermal enhancer-promoter interactions in ESCs
Recruitment of TAF3 at endodermal enhancers by CTCF and chromatin looping activates Mapk3 in ESCs. Apart from being a component of TFIID at core promoters, TAF3 may also associate with other transcription factors across the genome in ESCs. For instance, TAF3 represses the activity of pluripotency-associated transcription factors (OCT4, SOX2 and NANOG).
Figure 5. CTCF regulates V(D)J recombination
V(D)J recombination at antigen receptor loci is regulated by chromatin accessibility, which correlates with active histone modifications and transcription. CTCF may influence the outcome of V(D)J recombination by regulating enhancer-promoter interactions and locus compaction. At the IgH locus, CTCF-mediated looping of DH-JH-CH segments imposes ordered recombination (DH-to-JH) by controlling the communication of enhancers (Eμ and 3'RR) with distinct gene segments. Binding of CTCF at IGCR1 blocks the influence of the Eμ enhancer on proximal VH segments and prevents the spread of active histone modification from DH into the proximal VH region. In addition, it inhibits the level of antisense transcription within the DH region and modulates locus compaction in collaboration with other factors (e.g. YY1, Ikaros, Pax5, E2A). As a consequence, CTCF within IGCR1 may bias the rearrangement of distal (over proximal) VH segments with DJH joins.
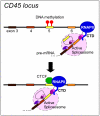
Figure 6. CTCF promotes alternative mRNA splicing
Mutually exclusive DNA methylation and CTCF binding may regulate alternative splicing. At the CD45 gene, DNA methylation at exon 5 inhibits CTCF binding, which leads to fairly unimpeded transcriptional elongation by RNA polymerase II (RNAPII) and subsequent exclusion of exon 5 during splicing of the resultant mRNA (upper panel). By contrast, hypomethylation of exon 5 leads to CTCF binding and RNAPII stalling, which promotes the inclusion of exon 5 (lower panel).
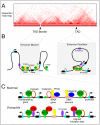
Figure 7. CTCF regulates three-dimensional genome architecture
A) Cartoon of an interaction heat map of a chromosome segment around 2.5 Mb in length depicting data generated by Hi-C in mammalian cells. The TADs and their borders are indicated. B) The presence of multiple CTCF and TFIIIC binding sites at TAD borders may contribute to the establishment of the border. This arrangement may explain the observed function of CTCF as an enhancer blocker. On the other hand, CTCF binding sites within TADs may facilitate enhancer-promoter looping through the recruitment of cohesin. The blue box denotes the promoter of the gene. C) Chromatin features of TAD borders in mammals and Drosophila melanogaster. The TAD borders in mammals are enriched for housekeeping and tRNA genes, SINE elements and CTCF binding sites. In D. melanogaster, they are enriched for highly transcribed genes and clusters of binding sites for various architectural proteins. The role of TFIIIC, cohesin and condensin proteins in mediating TAD border formation remains to be determined.
Similar articles
- Insulator function and topological domain border strength scale with architectural protein occupancy.
Van Bortle K, Nichols MH, Li L, Ong CT, Takenaka N, Qin ZS, Corces VG. Van Bortle K, et al. Genome Biol. 2014 Jun 30;15(6):R82. doi: 10.1186/gb-2014-15-5-r82. Genome Biol. 2014. PMID: 24981874 Free PMC article. - Direct Observation of Cell-Cycle-Dependent Interactions between CTCF and Chromatin.
Agarwal H, Reisser M, Wortmann C, Gebhardt JCM. Agarwal H, et al. Biophys J. 2017 May 23;112(10):2051-2055. doi: 10.1016/j.bpj.2017.04.018. Epub 2017 May 6. Biophys J. 2017. PMID: 28487148 Free PMC article. - Constitutively bound CTCF sites maintain 3D chromatin architecture and long-range epigenetically regulated domains.
Khoury A, Achinger-Kawecka J, Bert SA, Smith GC, French HJ, Luu PL, Peters TJ, Du Q, Parry AJ, Valdes-Mora F, Taberlay PC, Stirzaker C, Statham AL, Clark SJ. Khoury A, et al. Nat Commun. 2020 Jan 7;11(1):54. doi: 10.1038/s41467-019-13753-7. Nat Commun. 2020. PMID: 31911579 Free PMC article. - Architectural proteins, transcription, and the three-dimensional organization of the genome.
Cubeñas-Potts C, Corces VG. Cubeñas-Potts C, et al. FEBS Lett. 2015 Oct 7;589(20 Pt A):2923-30. doi: 10.1016/j.febslet.2015.05.025. Epub 2015 May 22. FEBS Lett. 2015. PMID: 26008126 Free PMC article. Review. - Differential 3D genome architecture and imprinted gene expression: cause or consequence?
Moindrot B, Imaizumi Y, Feil R. Moindrot B, et al. Biochem Soc Trans. 2024 Jun 26;52(3):973-986. doi: 10.1042/BST20230143. Biochem Soc Trans. 2024. PMID: 38775198 Free PMC article. Review.
Cited by
- Contribution of Topological Domains and Loop Formation to 3D Chromatin Organization.
Ea V, Baudement MO, Lesne A, Forné T. Ea V, et al. Genes (Basel). 2015 Jul 27;6(3):734-50. doi: 10.3390/genes6030734. Genes (Basel). 2015. PMID: 26226004 Free PMC article. Review. - TETology: Epigenetic Mastermind in Action.
Seethy A, Pethusamy K, Chattopadhyay I, Sah R, Chopra A, Dhar R, Karmakar S. Seethy A, et al. Appl Biochem Biotechnol. 2021 Jun;193(6):1701-1726. doi: 10.1007/s12010-021-03537-5. Epub 2021 Mar 10. Appl Biochem Biotechnol. 2021. PMID: 33694104 Review. - Coordinate regulation of ELF5 and EHF at the chr11p13 CF modifier region.
Swahn H, Sabith Ebron J, Lamar KM, Yin S, Kerschner JL, NandyMazumdar M, Coppola C, Mendenhall EM, Leir SH, Harris A. Swahn H, et al. J Cell Mol Med. 2019 Nov;23(11):7726-7740. doi: 10.1111/jcmm.14646. Epub 2019 Sep 26. J Cell Mol Med. 2019. PMID: 31557407 Free PMC article. - Phosphorylation of Tet3 by cdk5 is critical for robust activation of BRN2 during neuronal differentiation.
Rao VK, Swarnaseetha A, Tham GH, Lin WQ, Han BB, Benoukraf T, Xu GL, Ong CT. Rao VK, et al. Nucleic Acids Res. 2020 Feb 20;48(3):1225-1238. doi: 10.1093/nar/gkz1144. Nucleic Acids Res. 2020. PMID: 31807777 Free PMC article. - Integrating regulatory DNA sequence and gene expression to predict genome-wide chromatin accessibility across cellular contexts.
Nair S, Kim DS, Perricone J, Kundaje A. Nair S, et al. Bioinformatics. 2019 Jul 15;35(14):i108-i116. doi: 10.1093/bioinformatics/btz352. Bioinformatics. 2019. PMID: 31510655 Free PMC article.
References
- Baniahmad A, Steiner C, Kohne AC, Renkawitz R. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell. 1990;61:505–14. - PubMed
- Lobanenkov VV, et al. A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5'-flanking sequence of the chicken c-myc gene. Oncogene. 1990;5:1743–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources