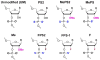2'-OMe-phosphorodithioate-modified siRNAs show increased loading into the RISC complex and enhanced anti-tumour activity - PubMed (original) (raw)
Xianbin Yang 2, Kshipra M Gharpure 3, Hiroto Hatakeyama 3, Martin Egli 4, Michael H McGuire 3, Archana S Nagaraja 3, Takahito M Miyake 3, Rajesha Rupaimoole 3, Chad V Pecot 5, Morgan Taylor 3, Sunila Pradeep 3, Malgorzata Sierant 6, Cristian Rodriguez-Aguayo 7, Hyun J Choi 3, Rebecca A Previs 3, Guillermo N Armaiz-Pena 3, Li Huang 8, Carlos Martinez 9, Tom Hassell 9, Cristina Ivan 10, Vasudha Sehgal 11, Richa Singhania 12, Hee-Dong Han 13, Chang Su 3, Ji Hoon Kim 14, Heather J Dalton 3, Chandra Kovvali 3, Khandan Keyomarsi 15, Nigel A J McMillan 16, Willem W Overwijk 17, Jinsong Liu 18, Ju-Seog Lee 11, Keith A Baggerly 19, Gabriel Lopez-Berestein 7, Prahlad T Ram 11, Barbara Nawrot 6, Anil K Sood 20
Affiliations
- PMID: 24619206
- PMCID: PMC4112501
- DOI: 10.1038/ncomms4459
2'-OMe-phosphorodithioate-modified siRNAs show increased loading into the RISC complex and enhanced anti-tumour activity
Sherry Y Wu et al. Nat Commun. 2014.
Abstract
Improving small interfering RNA (siRNA) efficacy in target cell populations remains a challenge to its clinical implementation. Here, we report a chemical modification, consisting of phosphorodithioate (PS2) and 2'-O-Methyl (2'-OMe) MePS2 on one nucleotide that significantly enhances potency and resistance to degradation for various siRNAs. We find enhanced potency stems from an unforeseen increase in siRNA loading to the RNA-induced silencing complex, likely due to the unique interaction mediated by 2'-OMe and PS2. We demonstrate the therapeutic utility of MePS2 siRNAs in chemoresistant ovarian cancer mouse models via targeting GRAM domain containing 1B (GRAMD1B), a protein involved in chemoresistance. GRAMD1B silencing is achieved in tumours following MePS2-modified siRNA treatment, leading to a synergistic anti-tumour effect in combination with paclitaxel. Given the previously limited success in enhancing siRNA potency with chemically modified siRNAs, our findings represent an important advance in siRNA design with the potential for application in numerous cancer types.
Conflict of interest statement
Conflict of interest
X.Y. is an employee of AM Biotechnologies LLC. The remaining authors declare no competing financial interests
Figures
Figure 1. Chemical structures of modified siRNAs
Chemical structures of modified siRNAs used for the initial screen.
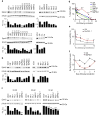
Figure 2. Stability and gene silencing activity of chemically modified siRNA
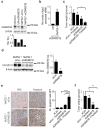
(a) Knockdown of EphA2 in SKOV3ip1 cells using chemically modified siRNAs (100 nM, serum-free condition). (b) Knockdown of EphA2 using selected siRNA sequences in HeyA8 cells (100 nM, serum-free condition). (c) Knockdown of EphA2 using chemically modified siRNAs in SKOV3ip1 cells (10, 20, and 50 nM, 10% FBS-containing media). (a, b, and c) Cells were treated with siRNAs for 4 h and EphA2 protein levels were examined 48 h post-transfection. (d) Stability of chemically modified siRNAs in 10% FBS at 37 °C. (e) Intracellular stability of UM and MePS2-1 modified siEphA2 in SKOV3ip1 cells (50 nM, serum-free condition). (f) Duration of EphA2 knockdown following UM and MePS2-1 modified siEphA2 treatment in SKOV3ip1 cells (50 nM, 10% FBS-containing media). [_P_-values obtained with Student’s _t_-test; *, P<0.05; **, P<0.01; ***, P<0.001; or ****, P<0.0001; compared to UM siEphA2 sequence; bars and error bars represent mean values and the corresponding S.E.M.s (n=3)].
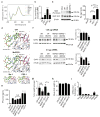
Figure 3. Biophysical properties of MePS2-1 siRNA
(a) Circular dichroism spectra of Me-1, PS-1, MePS-1, PS2-1, and MePS2-1 modified siEphA2s. (b) Intracellular binding of UM, PS2-1, MePS2-1, and Me2 modified siEphA2 to Ago2 protein in SKOV3ip1 cells (50 nM, serum-free condition). (c) Ago2 association with UM, PS2-1, and MePS2-1 biotin-labeled siRNAs. Apart from mock-treated (no treatment control) sample, each sample contains the same amount of siRNA (quantified using stem loop PCR). Transfection was performed using 50 nM biotin-labeled siRNA in serum-free condition. The bottom two panels show the effect of transfection on total Ago2 level in cells. (d) Computational modeling of the siRNA:PAZ interaction. UM, MePS2-1, Me-1, and PS2-1 modified siRNAs were modeled, all shown in the same orientation. (e) Induction of IFN-α by UM and MePS2-1 modified siEphA2 in C57BL/6-derived dendritic cells (75 nM, serum-free condition). A high IFN-α-inducing siRNA sequence and CpG2216 were used as positive controls. (f) Knockdown of EphA2 protein in tumors following a single dose of siRNA-DOPC treatment in SKOV3ip1 ovarian cancer mouse model (1.25 and 2.5 μg per dose). Effect of UM-siEphA2-DOPC and MePS2-1-siEphA2-DOPC on tumor burden (g) and body weight (h) in SKOV3ip1 orthotopic ovarian cancer mouse model following 4 weeks of siRNA treatment (n=10). (i) Biodistribution of MePS2-1-siEphA2-DOPC. SiRNA levels were measured in various organs at 24 h post intraperitoneal administration. Stemloop PCR technique was employed to assess intact siRNA levels. The total amount of siRNA in each organ was measured (n=4) and was expressed as percentage of injected dose (ID). [_P_-values obtained with Student’s _t_-test; (b) **, P<0.01; ****, P<0.0001; compared to UM; (c) ****, P<0.0001; compared to respective controls; (e) **, P<0.01; ***, P<0.001; compared to UM siCon; (f, g) *, P<0.05 or **, P<0.01; compared to the corresponding control groups; bars and error bars represent mean values and the corresponding S.E.M.s (n= 2-3)].
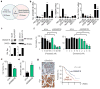
Figure 4. Targeting GRAMD1B for ovarian cancer treatment
(a) A Venn diagram for identification of novel targets for the treatment of ovarian cancer. (b) Expression of GRAMD1B, RBBP6, and SLC23A1, in a panel of chemosensitive and chemoresistant ovarian cancer cell lines. (c) Protein expression of GRAMD1B in taxane-sensitive (HeyA8 and SKOV3ip1) and taxane-resistant (HeyA8-MDR and SKOV3-TR) ovarian cancer cells (n=1). Effect of GRAMD1B downregulation on (d) cell viablity and (e) apoptosis in HeyA8-MDR cells. (f) Effect of knockdown of GRAMD1B on the sensitivity of HeyA8-MDR cells to paclitaxel or docetaxel treatment. Cell viability is expressed as a percentage of that measured in siRNA monotherapy treated wells. (g) Effect of GRAMD1B protein expression on overall survival in ovarian cancer patients (n=156). Scale bar represents 100 μm. [_P_-values obtained with (a-f) Student’s _t_-test or (g) log-rank test; (b) ***, P<0.001 or ****, P<0.0001; compared to the corresponding chemosensitive cell line; (d, e, f) *, P<0.05 or **, P<0.01; compared to cells treated with siCon; bars and error bars represent mean values and the corresponding S.E.M.s (n=3)].
Figure 5. Therapeutic effect of MePS2-1 siGRAMD1B in vitro and in vivo
(a) Knockdown of GRAMD1B using UM and MePS2-1 modified siRNAs (40 nM, 10% FBS-containing media) (n=1). (b) Intracellular binding of UM and MePS2-1 siGRAMD1B to Ago2 in HeyA8-MDR cells (50 nM, serum-free condition). (c) Effect of MePS2-1 siGRAMD1B on tumoral response to paclitaxel treatment in HeyA8-MDR orthotopic ovarian cancer mouse model. (d) Knockdown of GRMAD1B by MePS2-1-siGRAMD1B-DOPC in HeyA8-MDR tumors. (e) Caspase 3 activity in tumors treated with MePS2-1-siCon-DOPC or MePS2-1-siGRAMD1B-DOPC +/- paclitaxel. Scale bar represents 100 μm. (f) Effect of MePS2-1 siGRAMD1B on tumoral response to paclitaxel treatment in SKOV3-TR orthotopic ovarian cancer mouse model. [_P_-values obtained with Student’s _t_-test; (b) ***, P<0.001, compared to UM si GRAMD1B (n=3); (c, f) *, P<0.05 or **, P<0.01, compared to the corresponding control groups (n=10); (d) **, P<0.01, compared to MePS2-1-siCon-DOPC group (n=2-3); (e), ****, P<0.0001, compared to paclitaxel or MePS2-1-siGRAMD1B-DOPC monotherapy groups (n=10); bars and error bars represent mean values and the corresponding S.E.M.s].
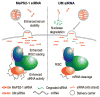
Figure 6. A schematic representation of mechanisms by which MePS2 modification enhances siRNA activity
MePS2-1 modification enhances serum stabilty of siRNAs and promotes the loading of the antisense strand into RISC.
Similar articles
- Competition for RISC binding predicts in vitro potency of siRNA.
Koller E, Propp S, Murray H, Lima W, Bhat B, Prakash TP, Allerson CR, Swayze EE, Marcusson EG, Dean NM. Koller E, et al. Nucleic Acids Res. 2006;34(16):4467-76. doi: 10.1093/nar/gkl589. Epub 2006 Aug 31. Nucleic Acids Res. 2006. PMID: 16945958 Free PMC article. - Gene silencing activity of siRNA molecules containing phosphorodithioate substitutions.
Yang X, Sierant M, Janicka M, Peczek L, Martinez C, Hassell T, Li N, Li X, Wang T, Nawrot B. Yang X, et al. ACS Chem Biol. 2012 Jul 20;7(7):1214-20. doi: 10.1021/cb300078e. Epub 2012 Apr 26. ACS Chem Biol. 2012. PMID: 22512638 Free PMC article. - A large-scale chemical modification screen identifies design rules to generate siRNAs with high activity, high stability and low toxicity.
Bramsen JB, Laursen MB, Nielsen AF, Hansen TB, Bus C, Langkjaer N, Babu BR, Højland T, Abramov M, Van Aerschot A, Odadzic D, Smicius R, Haas J, Andree C, Barman J, Wenska M, Srivastava P, Zhou C, Honcharenko D, Hess S, Müller E, Bobkov GV, Mikhailov SN, Fava E, Meyer TF, Chattopadhyaya J, Zerial M, Engels JW, Herdewijn P, Wengel J, Kjems J. Bramsen JB, et al. Nucleic Acids Res. 2009 May;37(9):2867-81. doi: 10.1093/nar/gkp106. Epub 2009 Mar 12. Nucleic Acids Res. 2009. PMID: 19282453 Free PMC article. - Exploring chemical modifications for siRNA therapeutics: a structural and functional outlook.
Shukla S, Sumaria CS, Pradeepkumar PI. Shukla S, et al. ChemMedChem. 2010 Mar 1;5(3):328-49. doi: 10.1002/cmdc.200900444. ChemMedChem. 2010. PMID: 20043313 Review. - [Components and assembly of RNA-induced silencing complex].
Song XM, Yan F, Du LX. Song XM, et al. Yi Chuan. 2006 Jun;28(6):761-6. Yi Chuan. 2006. PMID: 16818443 Review. Chinese.
Cited by
- Opportunities and challenges for the clinical translation of structured DNA assemblies as gene therapeutic delivery and vaccine vectors.
Dobrovolskaia MA, Bathe M. Dobrovolskaia MA, et al. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021 Jan;13(1):e1657. doi: 10.1002/wnan.1657. Epub 2020 Jul 15. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021. PMID: 32672007 Free PMC article. Review. - Amide linkages mimic phosphates in RNA interactions with proteins and are well tolerated in the guide strand of short interfering RNAs.
Mutisya D, Hardcastle T, Cheruiyot SK, Pallan PS, Kennedy SD, Egli M, Kelley ML, Smith AVB, Rozners E. Mutisya D, et al. Nucleic Acids Res. 2017 Aug 21;45(14):8142-8155. doi: 10.1093/nar/gkx558. Nucleic Acids Res. 2017. PMID: 28854734 Free PMC article. - A Highlight of Recent Advances in Aptamer Technology and Its Application.
Sun H, Zu Y. Sun H, et al. Molecules. 2015 Jun 30;20(7):11959-80. doi: 10.3390/molecules200711959. Molecules. 2015. PMID: 26133761 Free PMC article. Review. - X-aptamers targeting Thy-1 membrane glycoprotein in pancreatic ductal adenocarcinoma.
Wang H, Li X, Lai LA, Brentnall TA, Dawson DW, Kelly KA, Chen R, Pan S. Wang H, et al. Biochimie. 2021 Feb;181:25-33. doi: 10.1016/j.biochi.2020.11.018. Epub 2020 Nov 23. Biochimie. 2021. PMID: 33242496 Free PMC article. - Small interfering RNA for cancer treatment: overcoming hurdles in delivery.
Charbe NB, Amnerkar ND, Ramesh B, Tambuwala MM, Bakshi HA, Aljabali AAA, Khadse SC, Satheeshkumar R, Satija S, Metha M, Chellappan DK, Shrivastava G, Gupta G, Negi P, Dua K, Zacconi FC. Charbe NB, et al. Acta Pharm Sin B. 2020 Nov;10(11):2075-2109. doi: 10.1016/j.apsb.2020.10.005. Epub 2020 Oct 13. Acta Pharm Sin B. 2020. PMID: 33304780 Free PMC article. Review.
References
- Tabernero J, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–417. - PubMed
- Wu S, et al. Systemic delivery of E6/7 siRNA using novel lipidic particles and its application with cisplatin in cervical cancer mouse models. Gene Ther. 2011;18:14–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- U24 CA143835/CA/NCI NIH HHS/United States
- R01 CA152228/CA/NCI NIH HHS/United States
- UH2 TR000943-01/TR/NCATS NIH HHS/United States
- UH2 TR000943/TR/NCATS NIH HHS/United States
- R01 CA109298/CA/NCI NIH HHS/United States
- U54 CA151668/CA/NCI NIH HHS/United States
- R44 GM084552/GM/NIGMS NIH HHS/United States
- U24CA143835/CA/NCI NIH HHS/United States
- P50 CA083639/CA/NCI NIH HHS/United States
- R43 GM086937/GM/NIGMS NIH HHS/United States
- GM086937-01/GM/NIGMS NIH HHS/United States
- R01 CA087548/CA/NCI NIH HHS/United States
- T32 CA101642/CA/NCI NIH HHS/United States
- CA109298/CA/NCI NIH HHS/United States
- CA128797/CA/NCI NIH HHS/United States
- CA016672/CA/NCI NIH HHS/United States
- GM084552-04/GM/NIGMS NIH HHS/United States
- P50 CA098258/CA/NCI NIH HHS/United States
- R43 GM100777/GM/NIGMS NIH HHS/United States
- GM100777-01/GM/NIGMS NIH HHS/United States
- R44 GM086937/GM/NIGMS NIH HHS/United States
- R43 GM084552/GM/NIGMS NIH HHS/United States
- R01 CA128797/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources