Cardiovascular effects of phentermine and topiramate: a new drug combination for the treatment of obesity - PubMed (original) (raw)
Review
Cardiovascular effects of phentermine and topiramate: a new drug combination for the treatment of obesity
Jens Jordan et al. J Hypertens. 2014 Jun.
Free PMC article
Abstract
Weight loss can reduce the increased cardiovascular risk associated with obesity. Pharmacotherapy is a recognized weight loss treatment option; however, cardiovascular safety issues with some previous weight loss drugs raise concerns for newly approved pharmacotherapies. Phentermine is approved for short-term obesity treatment in conjunction with lifestyle modifications, but is commonly used chronically. Topiramate, approved for treating epilepsy and preventing migraines, also induces weight loss. A single-dose combination of low-dose phentermine and topiramate extended-release was recently approved by the United States Food and Drug Administration as an adjunct to lifestyle intervention for the chronic treatment of overweight/obese adults. This review summarizes and evaluates the cardiovascular risk/benefit profile associated with phentermine and topiramate, individually and in combination. Cardiovascular data associated with long-term use of phentermine and topiramate extended-release indicate that this combination may be a safe and effective option for reducing weight in overweight/obese patients at low-to-intermediate cardiovascular risk.
Figures
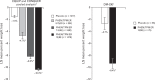
FIGURE 1
Weight loss from baseline to week 56 (1-year studies; ITT-LOCF) in overweight and obese adults with and without weight-related comorbidities (EQUIP, CONQUER) and in adults with T2DM (DM-230) . ∗P < 0.0001 vs. placebo. †Based on ITT-LOCF data from studies OB-302 and OB-303. ‡Based on ITT-LOCF data from study DM-230. ITT, intention to treat; LOCF, last observation carried forward; LS, least squares; PHEN/TPM-ER, phentermine and topiramate extended-release; T2DM, type 2 diabetes mellitus.
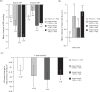
FIGURE 2
Changes in (a) blood pressure, (b) heart rate, and (c) rate pressure product from baseline to week 56 (1-year cohort; safety set) . Rate pressure product was defined as the product of the heart rate and SBP, divided by 1000. ∗P < 0.0001 vs. placebo. †P < 0.005 vs. placebo. This cardiovascular effects analysis includes patients with baseline and endpoint measurements. BP, blood pressure; PHEN/TPM-ER, phentermine and topiramate extended-release.
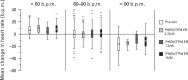
FIGURE 3
Effects on heart rate at week 56 based on baseline heart rate (1-year cohort; safety set) . b.p.m., beats per minute; PHEN/TPM-ER, phentermine and topiramate extended-release.
References
- World Health Organization Obesity and overweight fact sheet No 311. (WWW document); 2013. http://www.who.int/mediacentre/factsheets/fs311/en/index.html
- Nguyen T, Lau DC. The obesity epidemic and its impact on hypertension. Can J Cardiol 2012; 28:326–333 - PubMed
- Jordan J, Yumuk V, Schlaich M, Nilsson PM, Zahorska-Markiewicz B, Grassi G, et al. Joint statement of the European Association for the Study of Obesity and the European Society of Hypertension: obesity and difficult to treat arterial hypertension. J Hypertens 2012; 30:1047–1055 - PubMed
- Gerber Y, Jacobsen SJ, Frye RI, Weston SA, Killian JM, Roger VL. Secular trends in deaths from cardiovascular diseases: a 25-year community study. Circulation 2006; 113:2285–2292 - PubMed
- Sytkowski PA, Kannel WB, D’Agostino RB. Changes in risk factors and the decline in mortality from CVD. The Framingham Heart Study. N Engl J Med 1990; 322:1635–1641 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical