Monocyte-derived dendritic cells from Crohn's disease patients exhibit decreased ability to activate T helper type 17 responses in memory cells - PubMed (original) (raw)
Monocyte-derived dendritic cells from Crohn's disease patients exhibit decreased ability to activate T helper type 17 responses in memory cells
J K Nieminen et al. Clin Exp Immunol. 2014 Jul.
Abstract
An increased activation of interleukin (IL)-17A-producing immune cells is a well-established feature of Crohn's disease (CD). Mechanisms that contribute to this aberrant immune activation are, however, less clear. Given that an enhanced induction of innate-immunity associated cytokines IL-6 and IL-23, which promote IL-17 immunity, is also clearly implicated in CD, we hypothesized that monocyte-derived dendritic cells (moDCs) of CD patient origin would mount exaggerated IL-17A responses in T cells. However, we found a significantly attenuated up-regulation of the IL-17A response in allogeneic T helper memory cells in the presence of culture media from lipopolysaccharide (LPS)-stimulated moDCs of CD patients when compared with moDCs of control subjects (median fold-increase in IL-17A mRNA expression 1·09 versus 1·44, P = 0·038). This was accompanied by a lower expression of IL-1β and IL-6 transcripts in the LPS-treated moDCs (median 9·55 versus 13·9 relative units, P = 0·042, and 2·66 versus 9·06 relative units, P = 0·049, respectively). In addition, the up-regulation of autophagy-related LC3 transcripts was decreased in moDCs of CD patients (median fold-increase in mRNA expression 1·22 versus 1·52, P = 0·029). Our findings reveal similar immunological aberrancies in CD in the general population as reported in CD patients with mutated intracellular bacterial sensor NOD2, namely attenuated activation of innate cytokines and impaired autophagy, combined with a reduced capacity to up-regulate the T helper type 17 (Th17) response. The results presented here emphasize a defective anti-microbial response in the pathogenesis of CD. The increased mucosal Th1 and Th17 responses, which may contribute to the pathogenesis, could be the consequences of primary defects in the innate immunity.
Keywords: IL-1β; IL-6; Th17; dendritic cells; inflammatory bowel disease.
© 2014 British Society for Immunology.
Figures
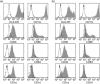
Fig. 1
Flow cytometric characterization of monocyte-derived dendritic cell (moDC) immunophenotype. CD14+ monocytes were magnetically enriched from fresh peripheral blood mononuclear cell (PBMC) fractions and differentiated to DCs by culturing for 5 days in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4. The appearance of the expected moDC immunophenotype was then evaluated by flow cytometric analysis; CD14 expression was profoundly down-regulated, while up-regulation of CD11c, CD80, CD86 and CD40 was observed, with relatively high expression of HLA-DR. Cells also acquired a variable expression of CD1a, and a small subgroup typically began to exhibit dim expression of CD83, a marker for mature DCs. Data from a Crohn's disease (CD) patient (a) and a healthy control (b) are shown. Unstained histograms represent isotype controls.
Fig. 2
Monocyte-derived dendritic cells (moDCs) differentiated from Crohn's disease (CD) patients' monocytes show lower interleukin (IL)-1β and IL-6 mRNA expression when stimulated with lipopolysaccharide (LPS); 5-day-differentiated moDCs were either stimulated with LPS or peptidoglycan (PGN), or left untreated, for 18 h. After stimulation with LPS, moDCs from CD patients expressed lower levels of IL-1β and IL-6 than cells from control subjects. Of note, IL-1β responses in CD patients were down-regulated below the level of unstimulated moDCs, whereas in controls the IL-1β levels were equivalent to unstimulated cells at 18 h. For IL-6 mRNA, upregulation by LPS and PGN was seen instead in both groups at this relatively late time-point (necessitated by the need to achieve sufficient supernatant cytokine levels for T cell stimulation). Transforming growth factor (TGF)-β1 mRNA expression was clearly down-regulated in the stimulated moDCs, while IL-23p19 expression remained generally low in all experimental conditions, with undetectable levels in a large proportion of the samples. Horizontal lines indicate medians for each group. Neg = unstimulated samples.
Fig. 3
Cytokine levels in monocyte-derived dendritic cell (moDC) supernatants; 5-day-differentiated moDCs were cultured with the indicated stimuli or left untreated for 18 h. Concentrations of the indicated cytokines in the culture supernatants were determined by a bead-based flow cytometric immunoassay. No significant differences were observed between the Crohn's disease (CD) patients and control subjects. Horizontal lines indicate median levels. Neg = unstimulated samples.
Fig. 4
Expression of co-stimulatory molecules CD80 and inducible co-stimulator-ligand (ICOS-L) in monocyte-derived dendritic cells (moDCs). mRNA expression for the co-stimulatory molecules CD80 and ICOS-L was assessed by reverse transcription–quantitative polymerase chain reaction (RT–qPCR) after 18 h culture of the 5-day-differentiated moDCs with the indicated stimuli. No significant differences were observed between the Crohn's disease (CD) patients and control subjects, although a trend existed for lower lipopolysaccharide (LPS)-induced CD80 up-regulation in patients. Horizontal lines indicate medians. Neg = unstimulated samples.
Fig. 5
Monocyte-derived dendritic cells (moDCs) from Crohn's disease (CD) patients exhibit weaker lipopolysaccharide (LPS)-induced up-regulation of autophagy marker LC3. mRNA expression for autophagy marker LC3 was measured by reverse transcription–quantitative polymerase chain reaction (RT–qPCR) after 18 h incubation of the 5-day-differentiated moDCs with LPS, peptidoglycan (PGN) or culture medium alone. Indicative of dysregulated autophagy induction, moDCs from CD patients showed weaker and non-significant up-regulation of LC3 expression when stimulated with LPS. PGN-induced up-regulation did not differ significantly from healthy controls. Horizontal lines in the upper plots indicate medians.
Fig. 6
Monocyte-derived dendritic cells (moDCs) of Crohn's disease (CD) patient origin have an attenuated capacity to up-regulate T helper type 17 (Th17) responses via soluble mediators upon lipopolysaccharide (LPS) stimulation. Allogeneic T helper memory cells (CD4+CD45RO+CD45RA–) were cultured with moDC-conditioned media of either CD patient or control subject origin (one-third of the total volume) in the presence of anti-CD3/CD28 beads for 6 days and then restimulated for 4 h with phorbol myristate acetate (PMA)+ionomycin prior to mRNA expression analysis by reverse transcription–quantitative polymerase chain reaction (RT–qPCR). In the absence of moDC stimulation (= neg), the mRNA levels for interleukin (IL)-17A, interferon (IFN)-γ, IL-4 or IL-10 in T cells did not significantly differ between the CD patients and control subjects. With LPS stimulation, moDC-conditioned media of CD patient origin induced less up-regulation of IL-17A and more of IL-10 than media prepared with control subject moDCs. In the lower plots, the effect of moDC stimuli on the expression of each cytokine mRNA in T cells is illustrated separately in CD patient and control subject groups (here the _P_-values are from Wilcoxon's signed-rank test, and the dashed lines indicate the mean mRNA expression levels in T cells in the absence of any moDC supernatant). Horizontal lines indicate medians for each group. Data are means from two experiments.
Fig. 7
The levels of cytokines in the supernatants from T cell cultures with moDC-conditioned media. Allogeneic T helper memory cells were cultured with monocyte-derived dendritic cell (moDC)-conditioned media (one-third of the total volume) in the presence of anti-CD3/CD28 beads for 6 days, then restimulated for 4 h with phorbol myristate acetate (PMA)+ionomycin, and supernatants were collected for the analysis of cytokine levels with a bead-based immunoassay. With both lipopolysaccharide (LPS) and peptidoglycan (PGN) stimulation, interleukin (IL)-17A protein seemed to be less up-regulated with moDC-conditioned media of Crohn's disease (CD) patient origin, in comparison with media obtained with cells from control subjects, although statistical significance was not observed. IL-2 up-regulation with PGN stimulation of moDCs was decreased in CD. However, a trend existed for a greater IL-2 protein induction by the unstimulated moDCs from CD patients, which might have influenced this finding. The direct contribution of moDC-derived IL-10 to the total IL-10 levels was relatively low and was not taken into consideration.
Similar articles
- Enrichment of IL-17A+ IFN-γ+ and IL-22+ IFN-γ+ T cell subsets is associated with reduction of NKp44+ ILC3s in the terminal ileum of Crohn's disease patients.
Li J, Doty AL, Tang Y, Berrie D, Iqbal A, Tan SA, Clare-Salzler MJ, Wallet SM, Glover SC. Li J, et al. Clin Exp Immunol. 2017 Oct;190(1):143-153. doi: 10.1111/cei.12996. Epub 2017 Jul 7. Clin Exp Immunol. 2017. PMID: 28586085 Free PMC article. - Monocyte-derived dendritic cells from Crohn patients show differential NOD2/CARD15-dependent immune responses to bacteria.
Salucci V, Rimoldi M, Penati C, Sampietro GM, van Duist MM, Matteoli G, Saibeni S, Vecchi M, Ardizzone S, Porro GB, Rescigno M. Salucci V, et al. Inflamm Bowel Dis. 2008 Jun;14(6):812-8. doi: 10.1002/ibd.20390. Inflamm Bowel Dis. 2008. PMID: 18240302 - TLR-stimulated CD34 stem cell-derived human skin-like and monocyte-derived dendritic cells fail to induce Th17 polarization of naive T cells but do stimulate Th1 and Th17 memory responses.
Duraisingham SS, Hornig J, Gotch F, Patterson S. Duraisingham SS, et al. J Immunol. 2009 Aug 15;183(4):2242-51. doi: 10.4049/jimmunol.0900474. Epub 2009 Jul 22. J Immunol. 2009. PMID: 19625644 - Innate and adaptive immunity in inflammatory bowel disease.
Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Geremia A, et al. Autoimmun Rev. 2014 Jan;13(1):3-10. doi: 10.1016/j.autrev.2013.06.004. Epub 2013 Jun 15. Autoimmun Rev. 2014. PMID: 23774107 Review. - Evidence from genetics for a role of autophagy and innate immunity in IBD pathogenesis.
Parkes M. Parkes M. Dig Dis. 2012;30(4):330-3. doi: 10.1159/000338119. Epub 2012 Jul 12. Dig Dis. 2012. PMID: 22796792 Review.
Cited by
- Escherichia coli in chronic inflammatory bowel diseases: An update on adherent invasive Escherichia coli pathogenicity.
Martinez-Medina M, Garcia-Gil LJ. Martinez-Medina M, et al. World J Gastrointest Pathophysiol. 2014 Aug 15;5(3):213-27. doi: 10.4291/wjgp.v5.i3.213. World J Gastrointest Pathophysiol. 2014. PMID: 25133024 Free PMC article. Review. - NOD2 in monocytes negatively regulates macrophage development through TNFalpha.
Chauvin C, Alvarez-Simon D, Radulovic K, Boulard O, Laine W, Delacre M, Waldschmitt N, Segura E, Kluza J, Chamaillard M, Poulin LF. Chauvin C, et al. Front Immunol. 2023 Jun 21;14:1181823. doi: 10.3389/fimmu.2023.1181823. eCollection 2023. Front Immunol. 2023. PMID: 37415975 Free PMC article.
References
- Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. - PubMed
- Sakuraba A, Sato T, Kamada N, Kitazume M, Sugita A, Hibi T. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn's disease. Gastroenterology. 2009;137:1736–1745. - PubMed
- Holtta V, Klemetti P, Sipponen T, et al. IL-23/IL-17 immunity as a hallmark of Crohn's disease. Inflamm Bowel Dis. 2008;14:1175–1184. - PubMed
- Rovedatti L, Kudo T, Biancheri P, et al. Differential regulation of interleukin-17 and interferon-{gamma} production in inflammatory bowel disease. Gut. 2009;58:1629–1636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical