Toward a consensus on the mechanism of nuclear pore complex inheritance - PubMed (original) (raw)
Review
. 2014 Mar-Apr;5(2):97-102.
doi: 10.4161/nucl.28314. Epub 2014 Feb 25.
Affiliations
- PMID: 24637838
- PMCID: PMC4049925
- DOI: 10.4161/nucl.28314
Review
Toward a consensus on the mechanism of nuclear pore complex inheritance
C Patrick Lusk et al. Nucleus. 2014 Mar-Apr.
Abstract
Nuclear compartmentalization is achieved through the enclosure of the genome by the nuclear envelope; the nuclear envelope is perforated by nuclear pore complexes (NPCs), which form portals that control molecular exchange between the nucleus and cytoplasm. The number of NPCs per nucleus establishes a limit to the flux of molecules across the nuclear envelope and might directly impact genome organization and gene expression in a cell type specific manner. Mechanisms that control NPC number remain ill defined. Our recent study implicates a cytoplasmic pool of the nucleoporin Nsp1 as a factor that controls NPC number during the asymmetric division of budding yeast; Nsp1 acts to ensure that daughters inherit NPCs. We place our data within an emerging model of NPC inheritance in yeast and consider potential analogous mechanisms in multicellular eukaryotes, including the functional conservation of a cytoplasmic pool of Nsp1.
Keywords: actin; asymmetric division; budding yeast; diffusion barrier; nuclear envelope; nuclear pore complex; nuclear transport; nucleus.
Figures
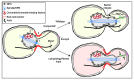
Figure 1. Mechanism of NPC inheritance in budding yeast. NPC dynamics and their segregation between mother and daughter cells are influenced by binding to cytoplasmic and nuclear structures including SPBs (1), and chromatin and/or chromatin binding partners like the LEM proteins (2). At least a subset of NPCs are likely attached to either a microtubule or actin-based cytoskeleton (3). There is a barrier at the bud neck (4) that impedes the passage of NPCs and other organelles, which likely responds to changes in cellular physiology. Under wild type conditions, Nsp1_CYT_ is translocated into the bud through a mechanism that requires an actin cytoskeleton and Myo2. Nsp1_CYT_ moves with ER tubules that extend from the mother nuclear envelope and contact the bud cortex; this ER might be connected to the cortex through the exocyst complex. The passage of Nsp1_CYT_ licenses NPC passage by contributing to the dissolution of the barrier (arrow). Under conditions in which there are disruptions to bud physiology and/or fitness, we propose that Nsp1_CYT_ function is inhibited, the barrier remains intact and NPCs are not transmitted.
Comment on
- Colombi P, Webster BM, Fröhlich F, Lusk CP. The transmission of nuclear pore complexes to daughter cells requires a cytoplasmic pool of Nsp1. J Cell Biol. 2013;203:215–32. doi: 10.1083/jcb.201305115.
Similar articles
- The transmission of nuclear pore complexes to daughter cells requires a cytoplasmic pool of Nsp1.
Colombi P, Webster BM, Fröhlich F, Lusk CP. Colombi P, et al. J Cell Biol. 2013 Oct 28;203(2):215-32. doi: 10.1083/jcb.201305115. J Cell Biol. 2013. PMID: 24165936 Free PMC article. - Inheritance of yeast nuclear pore complexes requires the Nsp1p subcomplex.
Makio T, Lapetina DL, Wozniak RW. Makio T, et al. J Cell Biol. 2013 Oct 28;203(2):187-96. doi: 10.1083/jcb.201304047. J Cell Biol. 2013. PMID: 24165935 Free PMC article. - The nuclear pore complex: a strategic platform for regulating cell signaling.
Gu Y. Gu Y. New Phytol. 2018 Jul;219(1):25-30. doi: 10.1111/nph.14756. Epub 2017 Aug 31. New Phytol. 2018. PMID: 28858378 Review. - Pom33, a novel transmembrane nucleoporin required for proper nuclear pore complex distribution.
Chadrin A, Hess B, San Roman M, Gatti X, Lombard B, Loew D, Barral Y, Palancade B, Doye V. Chadrin A, et al. J Cell Biol. 2010 May 31;189(5):795-811. doi: 10.1083/jcb.200910043. Epub 2010 May 24. J Cell Biol. 2010. PMID: 20498018 Free PMC article. - Structure and Assembly of the Nuclear Pore Complex.
Hampoelz B, Andres-Pons A, Kastritis P, Beck M. Hampoelz B, et al. Annu Rev Biophys. 2019 May 6;48:515-536. doi: 10.1146/annurev-biophys-052118-115308. Epub 2019 Apr 3. Annu Rev Biophys. 2019. PMID: 30943044 Review.
Cited by
- Surveillance of nuclear pore complex assembly by ESCRT-III/Vps4.
Webster BM, Colombi P, Jäger J, Lusk CP. Webster BM, et al. Cell. 2014 Oct 9;159(2):388-401. doi: 10.1016/j.cell.2014.09.012. Cell. 2014. PMID: 25303532 Free PMC article. - Nuclear envelope budding and its cellular functions.
Keuenhof KS, Kohler V, Broeskamp F, Panagaki D, Speese SD, Büttner S, Höög JL. Keuenhof KS, et al. Nucleus. 2023 Dec;14(1):2178184. doi: 10.1080/19491034.2023.2178184. Nucleus. 2023. PMID: 36814098 Free PMC article. - Border Safety: Quality Control at the Nuclear Envelope.
Webster BM, Lusk CP. Webster BM, et al. Trends Cell Biol. 2016 Jan;26(1):29-39. doi: 10.1016/j.tcb.2015.08.002. Epub 2015 Oct 1. Trends Cell Biol. 2016. PMID: 26437591 Free PMC article. Review.
References
- Weiner A, Dahan-Pasternak N, Shimoni E, Shinder V, von Huth P, Elbaum M, Dzikowski R. 3D nuclear architecture reveals coupled cell cycle dynamics of chromatin and nuclear pores in the malaria parasite Plasmodium falciparum. Cell Microbiol. 2011;13:967–77. doi: 10.1111/j.1462-5822.2011.01592.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases