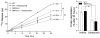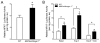MAFbx/Atrogin-1 is required for atrophic remodeling of the unloaded heart - PubMed (original) (raw)
MAFbx/Atrogin-1 is required for atrophic remodeling of the unloaded heart
Kedryn K Baskin et al. J Mol Cell Cardiol. 2014 Jul.
Abstract
Background: Mechanical unloading of the failing human heart induces profound cardiac changes resulting in the reversal of a distorted structure and function. In this process, cardiomyocytes break down unneeded proteins and replace those with new ones. The specificity of protein degradation via the ubiquitin proteasome system is regulated by ubiquitin ligases. Over-expressing the ubiquitin ligase MAFbx/Atrogin-1 in the heart inhibits the development of cardiac hypertrophy, but the role of MAFbx/Atrogin-1 in the unloaded heart is not known.
Methods and results: Mechanical unloading, by heterotopic transplantation, decreased heart weight and cardiomyocyte cross-sectional area in wild type mouse hearts. Unexpectedly, MAFbx/Atrogin-1(-/-) hearts hypertrophied after transplantation (n=8-10). Proteasome activity and markers of autophagy were increased to the same extent in WT and MAFbx/Atrogin-1(-/-) hearts after transplantation (unloading). Calcineurin, a regulator of cardiac hypertrophy, was only upregulated in MAFbx/Atrogin-1(-/-) transplanted hearts, while the mTOR pathway was similarly activated in unloaded WT and MAFbx/Atrogin-1(-/-) hearts. MAFbx/Atrogin-1(-/-) cardiomyocytes exhibited increased calcineurin protein expression, NFAT transcriptional activity, and protein synthesis rates, while inhibition of calcineurin normalized NFAT activity and protein synthesis. Lastly, mechanical unloading of failing human hearts with a left ventricular assist device (n=18) also increased MAFbx/Atrogin-1 protein levels and expression of NFAT regulated genes.
Conclusions: MAFbx/Atrogin-1 is required for atrophic remodeling of the heart. During unloading, MAFbx/Atrogin-1 represses calcineurin-induced cardiac hypertrophy. Therefore, MAFbx/Atrogin-1 not only regulates protein degradation, but also reduces protein synthesis, exerting a dual role in regulating cardiac mass.
Keywords: Atrophic remodeling; Heart assist device; Heterotopic heart transplantation; MAFbx/Atrogin-1; Protein turnover.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None of the authors has any financial conflicts of interest to disclose.
Disclosures
There are no financial conflicts of interest to report on behalf of the authors.
Figures
Figure 1
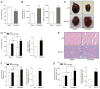
MAFbx/Atrogin-1 is required for atrophy during unloading of the heart by heterotopic transplantation. (A–B) Male wild type (WT) mice, ages 8–10 weeks, were subjected to heterotopic transplantation of the heart for 7 days (n = 5 per condition). (A) Heart weights of native and transplanted (unloaded) WT hearts. (B) MAFbx/Atrogin-1 and MuRF1 gene expression in native and transplanted (unloaded) WT hearts. (C–F) Male WT and MAFbx/Atrogin-1−/− littermate mice, ages 8–10 weeks, were subjected to isogenic heterotopic transplantation of the heart for 7 days (n = 8–10 per genotype and condition). (C) Representative native and transplanted (unloaded) hearts from WT and MAFbx/Atrogin-1−/− littermate mice. Scale bar: 1mm. (D) Heart weights and ratio of transplanted (unloaded) to native hearts. (E) Representative H&E staining of native and transplanted (unloaded) hearts from WT and MAFbx/Atrogin-1−/− littermate mice. Note the dense, small nuclei in the transplanted (unloaded), atrophied WT heart and the more sparsely distributed, large nuclei in the transplanted (unloaded) MAFbx/Atrogin-1−/− heart which is hypertrophied. Scale bar: 25μm. (F) Myocyte diameter and ratio, and (G) myocyte cross-sectional area and ratio of transplanted (unloaded) to native hearts. Data are mean ± SEM of 5–10 mice per condition. *P < 0.01 versus respective native. § P < 0.01 versus WT or WT transplanted (unloaded).
Figure 2
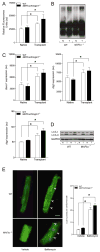
Proteasome activity and autophagy markers are increased in the transplanted (unloaded) heart. (A–D) Native and transplanted (unloaded) hearts from the WT and MAFbx/Atrogin-1−/− male mice described in Figure 1. (A) Proteasome (chymotryptic) activity and (B) polyubiquitinated proteins in native and transplanted (unloaded) WT and MAFbx/Atrogin-1−/− hearts. (C) mRNA expression of Becn1, Atg5, and Atg4, and (D) LC3-I and LC3-II protein levels in native and transplanted (unloaded) hearts. (E) Immunocytochemistry was performed on cardiomyocytes isolated from male WT and MAFbx/Atrogin-1−/− hearts (mice ages 8–10 weeks old) treated with Bafilomycin A. Arrowheads indicate examples of LC3-II puncta, representing autophagic vacuoles. Scale bar: 25μm. Quantification of LC3-II puncta is shown in the graph. Data are mean ± SEM. n = 8–10 per group (A–D) and n = 150–200 cells per condition (E). *P < 0.01 versus respective native or vehicle. N = native, T = transplanted (unloaded).
Figure 3
Protein degradation rates are not changed in MAFbx/Atrogin-1−/− cardiomyocytes. Cardiomyocytes were isolated from male WT and MAFbx/Atrogin-1−/− hearts (mice ages 8–10 weeks old) by Langendorff perfusion of enzyme-containing buffer. For protein degradation measurements, isolated myocytes were incubated for 24 hours with [14C]-phenylalanine (pulse), media was changed to contain unlabeled phenylalanine (chase), and release of [14C]-phenylalanine into the media was determined over time. Data are mean ± SEM. n = 3 independent experiments, 4–6 replicates per condition. *P < 0.01 versus respective control at the respective time points. # P<0.001linear regression of protein degradation rates.
Figure 4
Protein synthesis is increased in MAFbx/Atrogin-1 deficient cardiomyocytes, and is regulated by calcineurin. (A) Signaling pathways of protein synthesis are increased in the transplanted (unloaded) heart. Representative western blot analysis for mTOR, p70S6K, Akt and their respective phosphorylation status, in addition to eukaryotic initiation factor 3f (eIF3f) and calcineurin in native and transplanted (unloaded) WT and MAFbx/Atrogin-1−/− hearts. Data are mean ± SEM. n = 8–10 per group. *P < 0.05 versus WT Native. § P < 0.05 versus MAFbx/Atrogin-1−/− Native. #P < 0.05 versus WT transplanted (unloaded). CnA = calcineurin A, N = native, T = transplanted (unloaded). (B) Protein synthesis in WT and MAFbx/Atrogin-1−/− cardiomyocytes in control conditions and with inhibition of calcineurin or mTOR. For protein synthesis measurements, isolated myocytes were incubated with [14C]-phenylalanine and the incorporation of [14C]-phenylalanine into proteins was determined over time. Data are mean ± SEM. n = 3 independent experiments, 4–6 replicates per condition. # P < 0.01 versus all respective WT conditions at the respective time points. **P < 0.01 versus MAFbx/Atrogin-1−/− vehicle or treated at 16 hours. *P < 0.01 versus respective control or WT. § P < 0.05 versus MAFbx/Atrogin-1−/− Native. CsA = cyclosporine A.
Figure 5
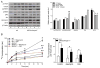
Calcineurin activity is increased in MAFbx/Atrogin-1 deficient cardiomyocytes. (A–B) Cardiomyocytes were isolated from male WT and MAFbx/Atrogin-1−/− hearts (mice ages 8–10 weeks old) and infected with NFAT-luciferase reporter adenovirus. In separate experiments infected myocytes were treated with angiotensin II and cyclosporine A. (A) NFAT-luciferase activity in isolated adult cardiomyocytes from WT and MAFbx/Atrogin-1−/− mice. (B) NFAT-luciferase activity in cardiomyocytes in response to stimulation. Data are mean ± SEM. n = 3 independent experiments, 7–9 replicates per condition. *P < 0.05 versus respective WT. AngII = angiotensin II; CsA = cyclosporin A.
Figure 6
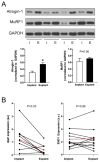
MAFbx/Atrogin-1 is increased in mechanically unloaded human hearts. (A) Protein expression and quantification at time of left ventricular assist device (LVAD) implantation (I) and explantation (E) in human patients diagnosed with idiopathic cardiomyopathy. Data are representative of n=11–13 paired human heart samples. (B) NFAT-regulated gene expression in human patients before and after LVAD placement. The red line indicates the average gene expression of implant and explant samples. BNP = brain natriuretic peptide. END1 = endothelin1.
Figure 7
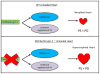
MAFbx/Atrogin-1 regulates protein synthesis in the transplanted (unloaded) heart. Under conditions of cardiac atrophy, such as heterotopic transplantation, MAFbx/Atrogin-1 keeps protein synthesis (PS) proportional to the rate of protein degradation (PD) by degrading calcineurin and other unidentified regulators of protein synthesis. Consequently, the heart atrophies (top panel). Heterotopic transplantation of the heart in the absence of MAFbx/Atrogin-1 leads to uncontrolled protein synthesis through calcineurin and perhaps other regulators of protein synthesis (bottom panel).
Similar articles
- Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex.
Li HH, Kedar V, Zhang C, McDonough H, Arya R, Wang DZ, Patterson C. Li HH, et al. J Clin Invest. 2004 Oct;114(8):1058-71. doi: 10.1172/JCI22220. J Clin Invest. 2004. PMID: 15489953 Free PMC article. - Transcriptional effects of E3 ligase atrogin-1/MAFbx on apoptosis, hypertrophy and inflammation in neonatal rat cardiomyocytes.
Zeng Y, Li J, Wang HX, Guo SB, Yang H, Zeng XJ, Fang Q, Tang CS, Du J, Li HH. Zeng Y, et al. PLoS One. 2013;8(1):e53831. doi: 10.1371/journal.pone.0053831. Epub 2013 Jan 15. PLoS One. 2013. PMID: 23335977 Free PMC article. - Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins.
Li HH, Willis MS, Lockyer P, Miller N, McDonough H, Glass DJ, Patterson C. Li HH, et al. J Clin Invest. 2007 Nov;117(11):3211-23. doi: 10.1172/JCI31757. J Clin Invest. 2007. PMID: 17965779 Free PMC article. - You spin me round: MaFBx/Atrogin-1 feeds forward on FOXO transcription factors (like a record).
Schisler JC, Willis MS, Patterson C. Schisler JC, et al. Cell Cycle. 2008 Feb 15;7(4):440-3. doi: 10.4161/cc.7.4.5451. Epub 2007 Dec 12. Cell Cycle. 2008. PMID: 18235241 Review. - Calcineurin in the heart: New horizons for an old friend.
Chaklader M, Rothermel BA. Chaklader M, et al. Cell Signal. 2021 Nov;87:110134. doi: 10.1016/j.cellsig.2021.110134. Epub 2021 Aug 25. Cell Signal. 2021. PMID: 34454008 Free PMC article. Review.
Cited by
- Targeting MuRF1 to Combat Skeletal Muscle Wasting in Cardiac Cachexia: Mechanisms and Therapeutic Prospects.
Liu X, Wen Y, Lu Y. Liu X, et al. Med Sci Monit. 2024 Oct 22;30:e945211. doi: 10.12659/MSM.945211. Med Sci Monit. 2024. PMID: 39434377 Free PMC article. Review. - Atrogin-1 promotes muscle homeostasis by regulating levels of endoplasmic reticulum chaperone BiP.
Ruparelia AA, Montandon M, Merriner J, Huang C, Wong SFL, Sonntag C, Hardee JP, Lynch GS, Miles LB, Siegel A, Hall TE, Schittenhelm RB, Currie PD. Ruparelia AA, et al. JCI Insight. 2024 Mar 26;9(8):e167578. doi: 10.1172/jci.insight.167578. JCI Insight. 2024. PMID: 38530354 Free PMC article. - Regression of cardiac hypertrophy in health and disease: mechanisms and therapeutic potential.
Martin TG, Juarros MA, Leinwand LA. Martin TG, et al. Nat Rev Cardiol. 2023 May;20(5):347-363. doi: 10.1038/s41569-022-00806-6. Epub 2023 Jan 4. Nat Rev Cardiol. 2023. PMID: 36596855 Free PMC article. Review. - Does Myocardial Atrophy Represent Anti-Arrhythmic Phenotype?
Bacova BS, Andelova K, Sykora M, Egan Benova T, Barancik M, Kurahara LH, Tribulova N. Bacova BS, et al. Biomedicines. 2022 Nov 4;10(11):2819. doi: 10.3390/biomedicines10112819. Biomedicines. 2022. PMID: 36359339 Free PMC article. Review. - Myostatin/AKT/FOXO Signaling Is Altered in Human Non-Ischemic Dilated Cardiomyopathy.
Hildebrandt L, Dieterlen MT, Klaeske K, Haunschild J, Saeed D, Eifert S, Borger MA, Jawad K. Hildebrandt L, et al. Life (Basel). 2022 Sep 12;12(9):1418. doi: 10.3390/life12091418. Life (Basel). 2022. PMID: 36143454 Free PMC article.
References
- Birks EJ. Molecular changes after left ventricular assist device support for heart failure. Circulation research. 2013;113:777–91. - PubMed
- Hellawell JL, Margulies KB. Myocardial reverse remodeling. Cardiovascular therapeutics. 2012;30:172–81. - PubMed
- Razeghi P, Myers TJ, Frazier OH, Taegtmeyer H. Reverse remodeling of the failing human heart with mechanical unloading. Emerging concepts and unanswered questions. Cardiology. 2002;98:167–74. - PubMed
- Razeghi P, Sharma S, Ying J, Li YP, Stepkowski S, Reid MB, et al. Atrophic remodeling of the heart in vivo simultaneously activates pathways of protein synthesis and degradation. Circulation. 2003;108:2536–41. - PubMed
- Razeghi P, Buksinska-Lisik M, Palanichamy N, Stepkowski S, Frazier OH, Taegtmeyer H. Transcriptional regulators of ribosomal biogenesis are increased in the unloaded heart. FASEB J. 2006;20:1090–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous