Microglia and motor neurons during disease progression in the SOD1G93A mouse model of amyotrophic lateral sclerosis: changes in arginase1 and inducible nitric oxide synthase - PubMed (original) (raw)
Microglia and motor neurons during disease progression in the SOD1G93A mouse model of amyotrophic lateral sclerosis: changes in arginase1 and inducible nitric oxide synthase
Katherine E Lewis et al. J Neuroinflammation. 2014.
Abstract
Background: Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease affecting the motor system. Although the etiology of the disease is not fully understood, microglial activation and neuroinflammation are thought to play a role in disease progression.
Methods: We examined the immunohistochemical expression of two markers of microglial phenotype, the arginine-metabolizing enzymes inducible nitric oxide synthase (iNOS) and arginase1 (Arg1), in the spinal cord of a mouse model carrying an ALS-linked mutant human superoxide dismutase transgene (SOD1(G93A)) and in non-transgenic wild-type (WT) mice. Immunolabeling for iNOS and Arg1 was evaluated throughout disease progression (6 to 25 weeks), and correlated with body weight, stride pattern, wire hang duration and ubiquitin pathology. For microglia and motor neuron counts at each time point, SOD1(G93A) and WT animals were compared using an independent samples t-test. A Welch t-test correction was applied if Levene's test showed that the variance in WT and SOD1G93A measurements was substantially different.
Results: Disease onset, measured as the earliest change in functional parameters compared to non-transgenic WT mice, occurred at 14 weeks of age in SOD1(G93A) mice. The ventral horn of the SOD1(G93A) spinal cord contained more microglia than WT from 14 weeks onwards. In SOD1(G93A) mice, Arg1-positive and iNOS-positive microglia increased 18-fold and 7-fold, respectively, between 10 and 25 weeks of age (endpoint) in the lumbar spinal cord, while no increase was observed in WT mice. An increasing trend of Arg1- and iNOS-expressing microglia was observed in the cervical spinal cords of SOD1(G93A) mice. Additionally, Arg1-negative motor neurons appeared to selectively decline in the spinal cord of SOD1(G93A) mice, suggesting that Arg1 may have a neuroprotective function.
Conclusions: This study suggests that the increase in spinal cord microglia occurs around and after disease onset and is preceded by cellular pathology. The results show that Arg1 and iNOS, thought to have opposing inflammatory properties, are upregulated in microglia during disease progression and that Arg1 in motor neurons may confer protection from disease processes. Further understanding of the neuroinflammatory response, and the Arg1/iNOS balance in motor neurons, may provide suitable therapeutic targets for ALS.
Figures
Figure 1
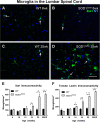
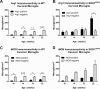
Anti-ionized calcium binding adaptor molecule 1 (Iba1) and tomato lectin labeling in amyotrophic lateral sclerosis-linked mutant human superoxide dismutase transgene (SOD1 G93A ) and wild-type (WT) lumbar spinal cord ventral horn. Few Iba1-positive microglia (arrows in A-D) were present in the ventral horn at 6 weeks of age in WT (A) and SOD1G93A (B) mice. The number of microglia was not increased at 25 weeks of age in WT mice (C), but was substantially increased at 25 weeks (disease endpoint, EP) in SOD1G93A mice (D). Microglial labeling with Iba1 (E) and tomato lectin (F) increased with disease progression. Scale bar 50 μm in A-D. *P < 0.05, **P < 0.01, ***P < 0.001. NY, Nuclear yellow.
Figure 2
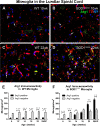
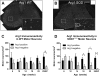
Arginase1 (Arg1) expression in microglia of amyotrophic lateral sclerosis-linked mutant human superoxide dismutase transgene (SOD1 G93A ) and wild-type (WT) lumbar spinal cord ventral horn. A subset of ventral horn tomato lectin (TL)-positive (red) microglia expressed Arg1 (green) (arrows, A-D) at 10 weeks of age in both WT (A) and SOD1G93A (B) mice. The number of microglia expressing Arg1 was unchanged at 22 weeks of age in WT mice (C); in contrast, 22-week SOD1G93A spinal cord (D) showed a far greater number of Arg1-positive microglia. The numbers of Arg1-negative and Arg1-positive microglia remained constant over time in WT mice (E). The number of Arg1-positive microglia increased with disease progression in SOD1G93A mice (F). Scale bar 50 μm in A-D. *P < 0.05, **P < 0.01, ***P < 0.001. NY, Nuclear yellow; EP, endpoint
Figure 3
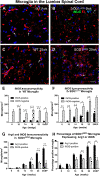
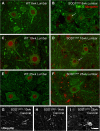
Inducible nitric oxide synthase expression in microglia of amyotrophic lateral sclerosis-linked mutant human superoxide dismutase transgene (SOD1 G93A ) and wild-type (WT) lumbar spinal cord ventral horn. The majority of tomato lectin (TL)-positive microglia (red) in the ventral horn did not express inducible nitric oxide synthase (iNOS) (arrows, A,B) at 6 weeks of age in either WT (A) or SOD1G93A (B) mice. A subset of microglia were iNOS-positive (green) (arrows, C,D). The numbers of iNOS-positive and iNOS-negative microglia stayed relatively constant over time in WT mice (E). The number of iNOS-positive microglia increased with disease progression in SOD1G93A mice; the number of iNOS-negative microglia increased at 22 and 25 weeks of age in SOD1G93A mice (F). The combined data from Figure 2F and 3F show that the number of both arginase1 (Arg1)-positive and iNOS-positive microglia increased with disease progression (G); the percentage of microglia expressing Arg1 and expressing iNOS also increased with time (H). Scale bar 50 μm in A-D. *P < 0.05, **P < 0.01, ***P < 0.001. NY, Nuclear yellow; EP, endpoint.
Figure 4
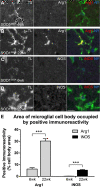
Percentage area occupied by arginase1 (Arg1) and inducible nitric oxide synthase (iNOS) immunoreactivity in amyotrophic lateral sclerosis-linked mutant human superoxide dismutase transgene (SOD1 G93A ) lumbar spinal cord microglia. The percentage area of the microglial cell body occupied by Arg1 (A,B) or iNOS (C,D) positive immunoreactivity was examined at 6 weeks (A,C) and 22 weeks (B,D) of age in a subset of microglia displaying the strongest immunoreactivity. The percentage of cell body area occupied by Arg1 was higher at 22 weeks of age than at 6 weeks of age; the percentage of cell body area occupied by iNOS was also higher at 22 weeks of age than at 6 weeks of age (E). Scale bar 10 μm in A-D. ***P < 0.01. TL, tomato lectin; NY, Nuclear yellow.
Figure 5
Expression of arginase1 (Arg1) and inducible nitric oxide synthase (iNOS) in microglia of the ventral horn of wild-type (WT) and amyotrophic lateral sclerosis-linked mutant human superoxide dismutase transgene (SOD1 G93A ) cervical spinal cord. The number of Arg1-negative and Arg1-positive cervical microglia remained relatively constant over time in WT mice (A). In the cervical spinal cord of SOD1G93A mice, the number of Arg1-positive and Arg1-negative microglia remained equivalent until 22 weeks of age, when there was an increase in the number of Arg1-positive microglia (B). There were greater numbers of iNOS-negative microglia than iNOS-positive microglia throughout the time course in WT mice (C). The numbers of iNOS-positive microglia and iNOS-negative microglia both increased with disease progression in the SOD1G93A cervical spinal cord (D). *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 6
Arginase1 (Arg1) expression in motor neurons of wild-type (WT) and amyotrophic lateral sclerosis-linked mutant human superoxide dismutase transgene (SOD1 G93A ) ventral horn. Arg1 immunostaining was observed in a punctate pattern in motor neuron cell bodies (A, inset). At 22 weeks of age, Arg1 labeling was far more prominent in WT motor neurons (A) than in SOD1G93A motor neurons (B). The majority of Arg1-positive cells in the 22-week SOD1G93A spinal cord were microglial cells (B; white box in inset). At 25 weeks of age, WT mice showed fewer Arg1-negative than Arg1-positive motor neurons (C). In SOD1G93A mice, there was an earlier decline in the number of Arg1-negative motor neurons, from approximately 18 weeks of age (D), with significantly fewer Arg1-negative than Arg1-positive motor neurons at 18, 22 and 25 weeks of age (disease endpoint, EP). Scale bar 300 μm in A and B, 130 μm in A (inset), 125 μm in B (inset). *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 7
Ubiquitin immunolabeling in amyotrophic lateral sclerosis-linked mutant human superoxide dismutase transgene (SOD1 G93A ) and wild-type (WT) mice. Ubiquitin (red, A-F) was localized to the nucleus in WT (A,C,E) and SOD1G93A**(B,D,F)** motor neurons (SMI32, green, A-F). In SOD1G93A mice lumbar spinal cord, intracellular ubiquitin-positive inclusions were present from 6 weeks of age (arrow, B) onwards. SOD1G93A mice also showed extra-neuronal ubiquitin-positive inclusions from 10 weeks of age (D), with numerous ubiquitin aggregates by 25 weeks of age (F). Additionally, vacuolated lumbar motor neurons were observed from 10 weeks of age (arrow, D). Intracellular ubiquitinated aggregates were observed at 10 weeks of age in cervical motor neurons (G, arrow); at 14 weeks of age, intracellular (H, arrow) and extracellular (H, arrowhead) ubiquitin-positive aggregates were present in the SOD1G93A cervical ventral horn, and increased in number with time (I, 22 weeks of age). Scale bar 12 μm in A-F, 20 μm in G-I.
Figure 8
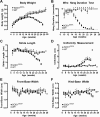
Functional measures of disease progression in wild-type (WT) and amyotrophic lateral sclerosis-linked mutant human superoxide dismutase transgene (SOD1 G93A ) mice. Body weight increased continuously in WT mice but followed a curved trajectory in SOD1G93A mice, becoming significantly less than that of WT mice at 14 weeks of age and reaching peak body weight at approximately 16 weeks of age (A). WT mice were able to maintain wire hang duration throughout the study, while SOD1G93A mice became unable to maintain a 60-second hang duration from 15 weeks of age onwards (B). SOD1G93A mice displayed a lower stride length (C) and a higher uniformity measure (D) than WT mice at 18 weeks and thereafter. Front-base width (E) and hind-base width (F) showed no consistent changes between WT and SOD1G93A mice over time. *P < 0.05,  P < 0.01, #P < 0.001.
P < 0.01, #P < 0.001.
Similar articles
- Ablation of P2X7 receptor exacerbates gliosis and motoneuron death in the SOD1-G93A mouse model of amyotrophic lateral sclerosis.
Apolloni S, Amadio S, Montilli C, Volonté C, D'Ambrosi N. Apolloni S, et al. Hum Mol Genet. 2013 Oct 15;22(20):4102-16. doi: 10.1093/hmg/ddt259. Epub 2013 Jun 4. Hum Mol Genet. 2013. PMID: 23736299 - Toll-Like Receptor-4 Inhibitor TAK-242 Attenuates Motor Dysfunction and Spinal Cord Pathology in an Amyotrophic Lateral Sclerosis Mouse Model.
Fellner A, Barhum Y, Angel A, Perets N, Steiner I, Offen D, Lev N. Fellner A, et al. Int J Mol Sci. 2017 Aug 1;18(8):1666. doi: 10.3390/ijms18081666. Int J Mol Sci. 2017. PMID: 28763002 Free PMC article. - Inducible nitric oxide synthase is present in motor neuron mitochondria and Schwann cells and contributes to disease mechanisms in ALS mice.
Chen K, Northington FJ, Martin LJ. Chen K, et al. Brain Struct Funct. 2010 Mar;214(2-3):219-34. doi: 10.1007/s00429-009-0226-4. Epub 2009 Nov 4. Brain Struct Funct. 2010. PMID: 19888600 Free PMC article. - New Insights on the Mechanisms of Disease Course Variability in ALS from Mutant SOD1 Mouse Models.
Nardo G, Trolese MC, Tortarolo M, Vallarola A, Freschi M, Pasetto L, Bonetto V, Bendotti C. Nardo G, et al. Brain Pathol. 2016 Mar;26(2):237-47. doi: 10.1111/bpa.12351. Brain Pathol. 2016. PMID: 26780365 Free PMC article. Review. - MicroRNA expression in animal models of amyotrophic lateral sclerosis and potential therapeutic approaches.
Martinez B, Peplow PV. Martinez B, et al. Neural Regen Res. 2022 Apr;17(4):728-740. doi: 10.4103/1673-5374.322431. Neural Regen Res. 2022. PMID: 34472458 Free PMC article. Review.
Cited by
- Human CD4+CD25+ T cells expressing a chimeric antigen receptor against aberrant superoxide dismutase 1 trigger antigen-specific immunomodulation.
Graber DJ, Cook WJ, Sentman ML, Murad-Mabaera JM, Sentman CL. Graber DJ, et al. Cytotherapy. 2024 Feb;26(2):126-135. doi: 10.1016/j.jcyt.2023.11.007. Epub 2023 Dec 3. Cytotherapy. 2024. PMID: 38043051 - Microglial depletion alters the brain neuroimmune response to acute binge ethanol withdrawal.
Walter TJ, Crews FT. Walter TJ, et al. J Neuroinflammation. 2017 Apr 20;14(1):86. doi: 10.1186/s12974-017-0856-z. J Neuroinflammation. 2017. PMID: 28427424 Free PMC article. - The Dual Role of Microglia in ALS: Mechanisms and Therapeutic Approaches.
Geloso MC, Corvino V, Marchese E, Serrano A, Michetti F, D'Ambrosi N. Geloso MC, et al. Front Aging Neurosci. 2017 Jul 25;9:242. doi: 10.3389/fnagi.2017.00242. eCollection 2017. Front Aging Neurosci. 2017. PMID: 28790913 Free PMC article. Review. - Fingolimod: A Disease-Modifier Drug in a Mouse Model of Amyotrophic Lateral Sclerosis.
Potenza RL, De Simone R, Armida M, Mazziotti V, Pèzzola A, Popoli P, Minghetti L. Potenza RL, et al. Neurotherapeutics. 2016 Oct;13(4):918-927. doi: 10.1007/s13311-016-0462-2. Neurotherapeutics. 2016. PMID: 27456702 Free PMC article.
References
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Van Den Bergh R, Hung W-Y, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-Vance MA, Haines J, Rouleau GA. et al.Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. - DOI - PubMed
- Borchelt DR, Lee MK, Slunt HS, Guarnieri M, Xu ZS, Wong PC, Brown RH Jr, Price DL, Sisodia SS, Cleveland DW. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc Natl Acad Sci U S A. 1994;91:8292–8296. doi: 10.1073/pnas.91.17.8292. - DOI - PMC - PubMed
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen W, Zhai P, Sufit RL, Siddique T. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous