Fatty acid synthase is required for mammary gland development and milk production during lactation - PubMed (original) (raw)
Fatty acid synthase is required for mammary gland development and milk production during lactation
Janel Suburu et al. Am J Physiol Endocrinol Metab. 2014.
Abstract
The mammary gland is one of the few adult tissues that strongly induce de novo fatty acid synthesis upon physiological stimulation, suggesting that fatty acid is important for milk production during lactation. The committed enzyme to perform this function is fatty acid synthase (FASN). To determine whether de novo fatty acid synthesis is obligatory or dietary fat is sufficient for mammary gland development and function during lactation, Fasn was specifically knocked out in mouse mammary epithelial cells. We found that deletion of Fasn hindered the development and induced the premature involution of the lactating mammary gland and significantly decreased medium- and long-chain fatty acids and total fatty acid contents in the milk. Consequently, pups nursing from Fasn knockout mothers experienced growth retardation and preweanling death, which was rescued by cross-fostering pups to a lactating wild-type mother. These results demonstrate that FASN is essential for the development, functional competence, and maintenance of the lactating mammary gland.
Keywords: fatty acid synthase; gene knockout; lactation; lipid synthesis; mammary gland.
Copyright © 2014 the American Physiological Society.
Figures
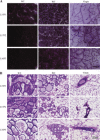
Fig. 1.
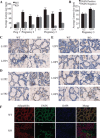
Epithelial cell-specific deletion of the fatty acid synthase gene (Fasn) in the mammary gland. Right inguinal mammary glands were harvested, sectioned, and stained by IHC for FASN. A: FASN knockout (KO) mothers showed only partial deletion of FASN following the first pregnancy, and further deletion in late lactation of the second and third pregnancies. Age-matched virgin glands showed very strong FASN staining in adipocytes and positive staining in the epithelium. Wild-type (WT) mothers showed strongly positive staining during all three pregnancies. B: deletion of FASN was heterogeneous and occurred gradually over the course of lactation. FASN-positive epithelial cells were present on lactation day L2 and L10 of the third pregnancy. By L16, nearly all epithelial cells were negative for FASN, and only adipocytes showed positive staining. All images are shown at ×20 resolution. LXPZ, lactation day X of pregnancy Z. Virgin glands are age-matched.
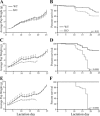
Fig. 2.
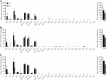
Deletion of FASN hinders growth and survival of nursing pups. Average weight of pups and their survival were monitored from L2 through L25 following the first (A), second (B), and third (C) pregnancies. Error bars represent SE. *P < 0.05, **P < 0.01, ***P < 0.001. P values for survival curves are indicated in the panel.
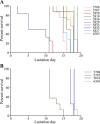
Fig. 3.
Preweanling death occurs as a whole litter. Survival of pups was monitored for each litter following the second (A) and third (B) pregnancies of KO mothers. Each line represents the survival of pups in a single litter from a KO mother. Typically, litters perished over the course of 2–3 days.
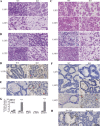
Fig. 4.
KO mammary glands are sparse in alveolar structures. A: left inguinal mammary glands were harvested and whole mounted at the indicated lactation day of each pregnancy. B: right inguinal mammary glands were harvested and stained with H&E at the indicated lactation day of each pregnancy. Virgin glands are age-matched.
Fig. 5.
FASN deletion induces early involution and cell death. Right inguinal mammary glands were harvested, sectioned, and stained for histology with H&E (A–C) or for cell death via TUNEL assay (D–F). Red arrows point to TUNEL-positive cells. G: TUNEL stains were quantified by counting the number of TUNEL-positive cells per mm2 of tissue. H: DNase I digestion was used as a positive TUNEL control, and no rTdT enzyme was used for a negative control. Error bars represent SE. ***P < 0.001.
Fig. 6.
Deletion of FASN hinders alveola. A: luminal measurements from WT and KO mice were averaged and compared for the respective lactation days of each pregnancy. B: measurements of FASN-positive lumens and FASN-negative lumens in KO mice were averaged and compared for L2P3 and L10P3. C–E: mammary gland sections from the first, second, and third pregnancies were stained for adipophilin as a marker of secretory activation, respectively. F: mammary glands from L10P3 were stained for adipophilin (red), FASN (green), and DAPI (blue). Error bars represent SE. ***P < 0.001.
Fig. 7.
FASN deletion changes the fatty acid profile of mammary gland milk. Milk was collected during the lactation period of each pregnancy. Milk was pooled from all 10 glands. Graphs represent fatty acid methyl ester analysis of the first (A), second (B), and third (C) pregnancies. All fatty acid values were normalized to total protein content. Error bars represent SE. *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 8.
Phenotypic rescue of offspring by cross-fostering mothers at birth. Litters from KO mothers were swapped with litters from WT mothers between L 1 and L3 for the first (A), second (B), and third (C) pregnancies. Average pup weight and survival were monitored every day beginning on the day after swapping mothers. Error bars represent SE. *P < 0.05, **P < 0.01, ***P < 0.001. P values for survival curves are indicated in the panel.
Similar articles
- Thyroid hormone responsive protein Spot14 enhances catalysis of fatty acid synthase in lactating mammary epithelium.
Rudolph MC, Wellberg EA, Lewis AS, Terrell KL, Merz AL, Maluf NK, Serkova NJ, Anderson SM. Rudolph MC, et al. J Lipid Res. 2014 Jun;55(6):1052-65. doi: 10.1194/jlr.M044487. Epub 2014 Apr 25. J Lipid Res. 2014. PMID: 24771867 Free PMC article. - Fatty acid synthase promoter: characterization, and transcriptional regulation by sterol regulatory element binding protein-1 in goat mammary epithelial cells.
Li J, Luo J, Xu H, Wang M, Zhu J, Shi H, Haile AB, Wang H, Sun Y. Li J, et al. Gene. 2015 Apr 25;561(1):157-64. doi: 10.1016/j.gene.2015.02.034. Epub 2015 Feb 14. Gene. 2015. PMID: 25688876 - The effects of cell death-inducing DNA fragmentation factor-α-like effector C (CIDEC) on milk lipid synthesis in mammary glands of dairy cows.
Yang Y, Lin Y, Duan X, Lv H, Xing W, Li Q, Gao X, Hou X. Yang Y, et al. J Dairy Sci. 2017 May;100(5):4014-4024. doi: 10.3168/jds.2016-11549. Epub 2017 Mar 9. J Dairy Sci. 2017. PMID: 28284693 - Milk lipid regulation at the maternal-offspring interface.
Yang D, Huynh H, Wan Y. Yang D, et al. Semin Cell Dev Biol. 2018 Sep;81:141-148. doi: 10.1016/j.semcdb.2017.10.012. Epub 2017 Oct 24. Semin Cell Dev Biol. 2018. PMID: 29051053 Free PMC article. Review. - Review: Mammary gland development in swine: embryo to early lactation.
Hurley WL. Hurley WL. Animal. 2019 Jul;13(S1):s11-s19. doi: 10.1017/S1751731119000521. Animal. 2019. PMID: 31280748 Review.
Cited by
- Fatty acid synthase (FASN) signalome: A molecular guide for precision oncology.
Menendez JA, Cuyàs E, Encinar JA, Vander Steen T, Verdura S, Llop-Hernández À, López J, Serrano-Hervás E, Osuna S, Martin-Castillo B, Lupu R. Menendez JA, et al. Mol Oncol. 2024 Mar;18(3):479-516. doi: 10.1002/1878-0261.13582. Epub 2024 Jan 18. Mol Oncol. 2024. PMID: 38158755 Free PMC article. - Involvement of the pro-oncogenic enzyme fatty acid synthase in the hallmarks of cancer: a promising target in anti-cancer therapies.
Vanauberg D, Schulz C, Lefebvre T. Vanauberg D, et al. Oncogenesis. 2023 Mar 18;12(1):16. doi: 10.1038/s41389-023-00460-8. Oncogenesis. 2023. PMID: 36934087 Free PMC article. Review. - Negative effects of long-term feeding of high-grain diets to lactating goats on milk fat production and composition by regulating gene expression and DNA methylation in the mammary gland.
Tian P, Luo Y, Li X, Tian J, Tao S, Hua C, Geng Y, Ni Y, Zhao R. Tian P, et al. J Anim Sci Biotechnol. 2017 Oct 1;8:74. doi: 10.1186/s40104-017-0204-2. eCollection 2017. J Anim Sci Biotechnol. 2017. PMID: 29026537 Free PMC article. - Fads3 modulates docosahexaenoic acid in liver and brain.
Zhang JY, Qin X, Liang A, Kim E, Lawrence P, Park WJ, Kothapalli KSD, Brenna JT. Zhang JY, et al. Prostaglandins Leukot Essent Fatty Acids. 2017 Aug;123:25-32. doi: 10.1016/j.plefa.2017.07.001. Epub 2017 Jul 8. Prostaglandins Leukot Essent Fatty Acids. 2017. PMID: 28838557 Free PMC article. - CCL2-Mediated Stromal Interactions Drive Macrophage Polarization to Increase Breast Tumorigenesis.
Archer M, Bernhardt SM, Hodson LJ, Woolford L, Van der Hoek M, Dasari P, Evdokiou A, Ingman WV. Archer M, et al. Int J Mol Sci. 2023 Apr 17;24(8):7385. doi: 10.3390/ijms24087385. Int J Mol Sci. 2023. PMID: 37108548 Free PMC article.
References
- Bartley JC, Emerman JT, Bissell MJ. Metabolic cooperativity between epithelial cells and adipocytes of mice. Am J Physiol Cell Physiol 241: C204–C208, 1981 - PubMed
- Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab 1: 309–322, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA163273/CA/NCI NIH HHS/United States
- T32 CA079448/CA/NCI NIH HHS/United States
- R01 CA107668/CA/NCI NIH HHS/United States
- R01 CA-107668/CA/NCI NIH HHS/United States
- R01 CA-163273/CA/NCI NIH HHS/United States
- P01 CA-106742/CA/NCI NIH HHS/United States
- P01 CA106742/CA/NCI NIH HHS/United States
- T32 CA-079448/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous