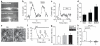Capillary pericytes regulate cerebral blood flow in health and disease - PubMed (original) (raw)
Capillary pericytes regulate cerebral blood flow in health and disease
Catherine N Hall et al. Nature. 2014.
Abstract
Increases in brain blood flow, evoked by neuronal activity, power neural computation and form the basis of BOLD (blood-oxygen-level-dependent) functional imaging. Whether blood flow is controlled solely by arteriole smooth muscle, or also by capillary pericytes, is controversial. We demonstrate that neuronal activity and the neurotransmitter glutamate evoke the release of messengers that dilate capillaries by actively relaxing pericytes. Dilation is mediated by prostaglandin E2, but requires nitric oxide release to suppress vasoconstricting 20-HETE synthesis. In vivo, when sensory input increases blood flow, capillaries dilate before arterioles and are estimated to produce 84% of the blood flow increase. In pathology, ischaemia evokes capillary constriction by pericytes. We show that this is followed by pericyte death in rigor, which may irreversibly constrict capillaries and damage the blood-brain barrier. Thus, pericytes are major regulators of cerebral blood flow and initiators of functional imaging signals. Prevention of pericyte constriction and death may reduce the long-lasting blood flow decrease that damages neurons after stroke.
Figures
Figure 1. Signalling pathways controlling capillary diameter
a Capillaries in the molecular layer of rat cerebellum labelled using isolectin B4; pericytes (arrow) labelled with NG2 or PDGFRβ antibodies. b Cerebellar capillaries in NG2-DsRed mouse; pericytes are red. c Neocortical capillaries in NG2-DsRed mouse; microglia labelled for Iba1. d Rat cerebellar capillary response to 2μM noradrenaline (NA) and superimposed 500μM glutamate (Glut). Line shows lumen diameter. e NA-evoked prolonged constriction (95% O2; a large constriction is shown for clarity). f Diameter in NA was not affected by [O2] (graphs show percentage of baseline diameter before drugs). g Glut dilates capillaries (20% O2; a large dilation is shown for clarity). h Dilation was larger in low [O2]. i NOS blocker L-NG-nitroarginine (L-NNA, 100μM) inhibits Glut-evoked dilation (20% O2). j Guanylyl cyclase blocker ODQ (10μM) does not block dilation (20% O2). k Blocking 20-HETE production (HET0016, 1μM) abolishes the inhibitory effect of L-NNA (20% O2). l L-NNA reduces Glut-evoked dilations at high and low [O2] (ANOVA p=0.002; p values from post-hoc t-tests). m ODQ does not affect Glut-evoked dilation. n HET0016 abolishes effect of L-NNA. o, p Blocking EP4 receptors (L161,982, 1μM) inhibits Glut-evoked dilation (o, 20% O2) at high and low [O2] (p). Data in d-p are from rat cerebellar capillaries. q EP4 block abolishes Glut-evoked dilation in rat neocortical capillaries (20% O2). Drug effects on baseline diameter are in Ext. Data Fig. 2.
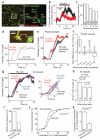
Figure 2. Pericyte membrane current and capillary dilation in cerebellar slices
a DsRed-labelled patch-clamped pericyte in molecular layer of mouse cerebellum. Lucifer yellow from pipette overlaps with DsRed. b Glutamate (Glut, 500μM) and NMDA (100μM) evoked outward current (−55mV). c Parallel fibre stimulation-evoked outward current (−74mV) is blocked by 1μM TTX. d Mean outward currents evoked by stimulation, glutamate and NMDA (b-d are in 95% O2). e-f Parallel fibre stimulation (in NA 1 μM) in rat cerebellar slice evokes capillary dilation (20% O2). g-h Constriction by NA (g) was unaffected by TTX or EP4 block (L161,982), which abolished the stimulation-evoked dilation (h). P values from one-way ANOVA with Dunnett’s post-hoc tests.
Figure 3. Active dilation of capillaries by pericytes in vivo in mouse cerebral cortex
a Confocal stack (90μm thick, maximum intensity projection) of FITC-dextran-filled vessels in vivo in somatosensory cortex of NG2-DsRed mouse (pericytes are red). Enlargement (single image) shows a penetrating arteriole (0th order) giving off a capillary (1st order) which splits into 2nd order branches. b Response of 45 capillary regions to 2s and 15s whisker pad stimulation. c Percentage of vessel regions of different orders (number studied on bars) showing >5% dilation to stimulation. d Simultaneous imaging (top, lines show measurement loci) of penetrating arteriole and 1st order capillary: capillary dilates 3s before arteriole (bottom: smoothing in d-g is explained in Methods and Ext. Data Fig. 4). e Dilation time course in simultaneously-imaged penetrating arterioles and 1st order capillaries. f Time to 10% of peak dilation for (j-1)th order (3rd order for j≥4) vessel minus that of jth order vessel. Capillaries dilate faster than arterioles. g Dilation time course in all responding (>5%) penetrating arterioles and 1st and 2nd order capillaries. Inset expands initial response. h Time to 10% of peak dilation in all 0th-2nd order responding vessels. i Percentage of capillary locations with or without pericytes showing >5% dilations. j Cumulative probability of capillary diameter changes (including “non-responding” capillaries with <5% dilations) in 464 pericyte and 168 non-pericyte locations. Diameter changes <0% (constrictions) represent random changes and measurement error. k Mean responses for distributions in j (p from Mann-Whitney U-test).
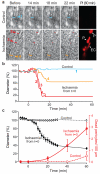
Figure 4. In ischaemia, pericytes constrict capillaries and then die in rigor
a Top: capillary in a rat cortical slice in normal solution. Bottom: capillary exposed to simulated ischaemia. Right: propidium iodide (PI) labelling after one hour, showing dead pericytes (P) and endothelial cell (EC). b Vessel diameters in a, at regions indicated, over time. c Mean diameter and number of dead pericytes/(100 μm) from 9 capillaries in ischaemia (measured at 18 locations) and 6 capillaries in normal solution (13 locations). Diameter in ischaemia was reduced from control (p=1.3×10−15 and 7.7.×10−17 at 30 and 60 mins). Pericyte death was higher in ischaemia at 40 and 60 mins (Mann-Whitney p=0.041 and 0.021). A few pericytes also died on capillaries in non-ischaemic slices, but did not constrict capillaries (1.3±1.5 % diameter decrease at 3 dead pericytes on 2 vessels).
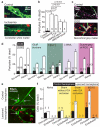
Figure 5. Pericyte death in ischaemia
a Rat cerebellar slice white matter capillaries labelled for NG2 and propidium iodide (PI) after 1 hour of control (white arrow: living pericyte) or ischaemia solution containing antimycin and iodoacetate (red arrow: dead pericyte). b Percentage of pericytes dead in control or after 1 hour’s ischaemia (as in a) alone or with block of action potentials (TTX, 1μM), AMPA/kainate receptors (25μM NBQX), or NMDA receptors (50μM D-AP5, 50μM MK-801, 100μM 7-chlorokynurenate); p values from one-way ANOVA with Dunnett’s post-hoc tests. c Rat neocortical slice grey matter capillaries labelled for IB4, NG2 and PI after 1 hour’s control solution or oxygen+glucose deprivation (OGD). d Percentage of pericytes (as in c) dead after one hour’s OGD (No-reoxy) or OGD followed by 1 hour of control solution (Reoxy) with no drugs, or with iGluR block (NBQX (25μM), AP5 (50μM) and 7CK (100μM)), zero [Ca2+]o, NOS block (100μM L-NNA), or free radical scavenging (150μM MnTBAP or 100μM PBN, pooled data from Ext. Data Fig. 5a) throughout. OGD killed pericytes (ANOVA, p=10−13) and death increased during reperfusion (p=3.3×10−13). iGluR block or zero [Ca2+]o reduced death (ANOVA with Dunnett’s post-hoc test, p=2.7×10−4 and 6.0×10−7). Blocking NOS had a small protective effect (p=0.026); ROS scavenging did not (p=0.99). e-f Confocal images of striatal capillaries labelled with IB4 and PI (e) and percentage of striatal pericytes and endothelial cells that are dead (f) from the control and lesioned hemisphere of in vivo MCAO-treated rats (90 mins, assessed 22.5 hours later), sham-operated rats (with or without filament being inserted into the internal carotid artery (ICA)), and naïve control animals. More pericytes die than endothelial cells (repeated measures ANOVA, p=10−6). For pericytes, but not endothelial cells, cell death is greater in lesioned hemisphere (main effect of hemisphere, p=0.004; hemisphere-cell type interaction p=0.003) and cell death is greater in MCAO-lesioned animals than in naïve or sham animals without ICA occlusion (Tukey post-hoc tests, p=0.005 and 0.01). See Ext. Data Fig. 5 for data from cortex.
Comment in
- Vascular biology: Brain vessels squeezed to death.
Greif DM, Eichmann A. Greif DM, et al. Nature. 2014 Apr 3;508(7494):50-1. doi: 10.1038/nature13217. Epub 2014 Mar 26. Nature. 2014. PMID: 24670635 No abstract available.
Similar articles
- Bidirectional control of CNS capillary diameter by pericytes.
Peppiatt CM, Howarth C, Mobbs P, Attwell D. Peppiatt CM, et al. Nature. 2006 Oct 12;443(7112):700-4. doi: 10.1038/nature05193. Epub 2006 Oct 1. Nature. 2006. PMID: 17036005 Free PMC article. - Active role of capillary pericytes during stimulation-induced activity and spreading depolarization.
Khennouf L, Gesslein B, Brazhe A, Octeau JC, Kutuzov N, Khakh BS, Lauritzen M. Khennouf L, et al. Brain. 2018 Jul 1;141(7):2032-2046. doi: 10.1093/brain/awy143. Brain. 2018. PMID: 30053174 Free PMC article. - Stimulation-induced increases in cerebral blood flow and local capillary vasoconstriction depend on conducted vascular responses.
Cai C, Fordsmann JC, Jensen SH, Gesslein B, Lønstrup M, Hald BO, Zambach SA, Brodin B, Lauritzen MJ. Cai C, et al. Proc Natl Acad Sci U S A. 2018 Jun 19;115(25):E5796-E5804. doi: 10.1073/pnas.1707702115. Epub 2018 Jun 4. Proc Natl Acad Sci U S A. 2018. PMID: 29866853 Free PMC article. - What is a pericyte?
Attwell D, Mishra A, Hall CN, O'Farrell FM, Dalkara T. Attwell D, et al. J Cereb Blood Flow Metab. 2016 Feb;36(2):451-5. doi: 10.1177/0271678X15610340. Epub 2015 Oct 14. J Cereb Blood Flow Metab. 2016. PMID: 26661200 Free PMC article. Review. - Diverse functions of pericytes in cerebral blood flow regulation and ischemia.
Fernández-Klett F, Priller J. Fernández-Klett F, et al. J Cereb Blood Flow Metab. 2015 Jun;35(6):883-7. doi: 10.1038/jcbfm.2015.60. Epub 2015 Apr 8. J Cereb Blood Flow Metab. 2015. PMID: 25853910 Free PMC article. Review.
Cited by
- Electroacupuncture of the trigeminal nerve causes _N_-methyl-D-aspartate receptors to mediate blood-brain barrier opening and induces neuronal excitatory changes.
Gong P, Zhang S, Ren L, Zhang J, Zhao Y, Mao X, Gan L, Wang H, Ma C, Lin Y, Ye Q, Qian K, Lin X. Gong P, et al. Front Cell Neurosci. 2022 Oct 13;16:1020644. doi: 10.3389/fncel.2022.1020644. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36313622 Free PMC article. - Vascular Homeostasis and Inflammation in Health and Disease-Lessons from Single Cell Technologies.
Bondareva O, Sheikh BN. Bondareva O, et al. Int J Mol Sci. 2020 Jun 30;21(13):4688. doi: 10.3390/ijms21134688. Int J Mol Sci. 2020. PMID: 32630148 Free PMC article. Review. - Progressive microvascular failure in acute ischemic stroke: A systematic review, meta-analysis, and time-course analysis.
Tudor T, Spinazzi EF, Alexander JE, Mandigo GK, Lavine SD, Grinband J, Connolly ES Jr. Tudor T, et al. J Cereb Blood Flow Metab. 2024 Feb;44(2):192-208. doi: 10.1177/0271678X231216766. Epub 2023 Nov 28. J Cereb Blood Flow Metab. 2024. PMID: 38016953 Free PMC article. - 20-Hydroxyeicosatetraenoic Acid Inhibition by HET0016 Offers Neuroprotection, Decreases Edema, and Increases Cortical Cerebral Blood Flow in a Pediatric Asphyxial Cardiac Arrest Model in Rats.
Shaik JS, Poloyac SM, Kochanek PM, Alexander H, Tudorascu DL, Clark RS, Manole MD. Shaik JS, et al. J Cereb Blood Flow Metab. 2015 Nov;35(11):1757-63. doi: 10.1038/jcbfm.2015.117. Epub 2015 Jun 10. J Cereb Blood Flow Metab. 2015. PMID: 26058691 Free PMC article. - A mouse model of small-vessel disease that produces brain-wide-identified microocclusions and regionally selective neuronal injury.
Silasi G, She J, Boyd JD, Xue S, Murphy TH. Silasi G, et al. J Cereb Blood Flow Metab. 2015 May;35(5):734-8. doi: 10.1038/jcbfm.2015.8. Epub 2015 Feb 18. J Cereb Blood Flow Metab. 2015. PMID: 25690472 Free PMC article.
References
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. - PubMed
Methods and Extended Data References
- Peters BP, Goldstein IJ. The use of fluorescein-conjugated Bandeiraea simplicifolia B4-isolectin as a histochemical reagent for the detection of alpha-D-galactopyranosyl groups. Their occurrence in basement membranes. Exp. Cell Res. 1979;120:321–334. - PubMed
- Laitinen L. Griffonia simplicifolia lectins bind specifically to endothelial cells and some epithelial cells in mouse tissues. Histochem. J. 1987;19:225–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources