A smartphone application to support recovery from alcoholism: a randomized clinical trial - PubMed (original) (raw)
Randomized Controlled Trial
doi: 10.1001/jamapsychiatry.2013.4642.
Fiona M McTavish 1, Ming-Yuan Chih 1, Amy K Atwood 1, Roberta A Johnson 1, Michael G Boyle 1, Michael S Levy 2, Hilary Driscoll 3, Steven M Chisholm 4, Lisa Dillenburg 1, Andrew Isham 1, Dhavan Shah 5
Affiliations
- PMID: 24671165
- PMCID: PMC4016167
- DOI: 10.1001/jamapsychiatry.2013.4642
Randomized Controlled Trial
A smartphone application to support recovery from alcoholism: a randomized clinical trial
David H Gustafson et al. JAMA Psychiatry. 2014 May.
Abstract
Importance: Patients leaving residential treatment for alcohol use disorders are not typically offered evidence-based continuing care, although research suggests that continuing care is associated with better outcomes. A smartphone-based application could provide effective continuing care.
Objective: To determine whether patients leaving residential treatment for alcohol use disorders with a smartphone application to support recovery have fewer risky drinking days than control patients.
Design, setting, and participants: An unmasked randomized clinical trial involving 3 residential programs operated by 1 nonprofit treatment organization in the Midwestern United States and 2 residential programs operated by 1 nonprofit organization in the Northeastern United States. In total, 349 patients who met the criteria for DSM-IV alcohol dependence when they entered residential treatment were randomized to treatment as usual (n = 179) or treatment as usual plus a smartphone (n = 170) with the Addiction-Comprehensive Health Enhancement Support System (A-CHESS), an application designed to improve continuing care for alcohol use disorders.
Interventions: Treatment as usual varied across programs; none offered patients coordinated continuing care after discharge. A-CHESS provides monitoring, information, communication, and support services to patients, including ways for patients and counselors to stay in contact. The intervention and follow-up period lasted 8 and 4 months, respectively.
Main outcomes and measures: Risky drinking days--the number of days during which a patient's drinking in a 2-hour period exceeded 4 standard drinks for men and 3 standard drinks for women, with standard drink defined as one that contains roughly 14 g of pure alcohol (12 oz of regular beer, 5 oz of wine, or 1.5 oz of distilled spirits). Patients were asked to report their risky drinking days in the previous 30 days on surveys taken 4, 8, and 12 months after discharge from residential treatment.
Results: For the 8 months of the intervention and 4 months of follow-up, patients in the A-CHESS group reported significantly fewer risky drinking days than did patients in the control group, with a mean of 1.39 vs 2.75 days (mean difference, 1.37; 95% CI, 0.46-2.27; P = .003).
Conclusions and relevance: The findings suggest that a multifeatured smartphone application may have significant benefit to patients in continuing care for alcohol use disorders.
Trial registration: clinicaltrials.gov Identifier: NCT01003119.
Figures
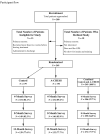
Figure
Participant flow.
Comment in
- Smartphone applications can help in treatment for alcoholism.
Andersson G. Andersson G. Evid Based Ment Health. 2015 Feb;18(1):27. doi: 10.1136/eb-2014-101927. Epub 2014 Sep 17. Evid Based Ment Health. 2015. PMID: 25231950 Free PMC article. No abstract available.
Similar articles
- Treatment seeking as a mechanism of change in a randomized controlled trial of a mobile health intervention to support recovery from alcohol use disorders.
Glass JE, McKay JR, Gustafson DH, Kornfield R, Rathouz PJ, McTavish FM, Atwood AK, Isham A, Quanbeck A, Shah D. Glass JE, et al. J Subst Abuse Treat. 2017 Jun;77:57-66. doi: 10.1016/j.jsat.2017.03.011. Epub 2017 Mar 30. J Subst Abuse Treat. 2017. PMID: 28476273 Free PMC article. Clinical Trial. - Effects of automated smartphone mobile recovery support and telephone continuing care in the treatment of alcohol use disorder: study protocol for a randomized controlled trial.
McKay JR, Gustafson DH, Ivey M, McTavish F, Pe-Romashko K, Curtis B, Oslin DA, Polsky D, Quanbeck A, Lynch KG. McKay JR, et al. Trials. 2018 Jan 30;19(1):82. doi: 10.1186/s13063-018-2466-1. Trials. 2018. PMID: 29382367 Free PMC article. - Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: a randomized clinical trial.
Bowen S, Witkiewitz K, Clifasefi SL, Grow J, Chawla N, Hsu SH, Carroll HA, Harrop E, Collins SE, Lustyk MK, Larimer ME. Bowen S, et al. JAMA Psychiatry. 2014 May;71(5):547-56. doi: 10.1001/jamapsychiatry.2013.4546. JAMA Psychiatry. 2014. PMID: 24647726 Free PMC article. Clinical Trial. - Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users.
Klimas J, Tobin H, Field CA, O'Gorman CS, Glynn LG, Keenan E, Saunders J, Bury G, Dunne C, Cullen W. Klimas J, et al. Cochrane Database Syst Rev. 2014 Dec 3;(12):CD009269. doi: 10.1002/14651858.CD009269.pub3. Cochrane Database Syst Rev. 2014. PMID: 25470303 Updated. Review. - Psychosocial interventions for people with both severe mental illness and substance misuse.
Hunt GE, Siegfried N, Morley K, Sitharthan T, Cleary M. Hunt GE, et al. Cochrane Database Syst Rev. 2013 Oct 3;(10):CD001088. doi: 10.1002/14651858.CD001088.pub3. Cochrane Database Syst Rev. 2013. PMID: 24092525 Updated. Review.
Cited by
- Rigid reduced successor representation as a potential mechanism for addiction.
Shimomura K, Kato A, Morita K. Shimomura K, et al. Eur J Neurosci. 2021 Jun;53(11):3768-3790. doi: 10.1111/ejn.15227. Epub 2021 May 10. Eur J Neurosci. 2021. PMID: 33840120 Free PMC article. - Therapeutic Content of Mobile Phone Applications for Substance Use Disorders: An Umbrella Review.
Oesterle TS, Hall-Flavin DK, Bormann NL, Loukianova LL, Fipps DC, Breitinger SA, Gilliam WP, Wu T, da Costa SC, Arndt S, Karpyak VM. Oesterle TS, et al. Mayo Clin Proc Digit Health. 2024 Jun;2(2):192-206. doi: 10.1016/j.mcpdig.2024.03.004. Epub 2024 Apr 11. Mayo Clin Proc Digit Health. 2024. PMID: 38983444 Free PMC article. - REMIT: Development of a mHealth theory-based intervention to decrease heavy episodic drinking among college students.
Kazemi DM, Borsari B, Levine MJ, Lamberson KA, Dooley B. Kazemi DM, et al. Addict Res Theory. 2018;26(5):377-385. doi: 10.1080/16066359.2017.1420783. Epub 2017 Dec 26. Addict Res Theory. 2018. PMID: 32694964 Free PMC article. - NIATx-TI versus typical product training on e-health technology implementation: a clustered randomized controlled trial study protocol.
White VM, Molfenter T, Gustafson DH, Horst J, Greller R, Gustafson DH Jr, Kim JS, Preuss E, Cody O, Pisitthakarm P, Toy A. White VM, et al. Implement Sci. 2020 Oct 23;15(1):94. doi: 10.1186/s13012-020-01053-4. Implement Sci. 2020. PMID: 33097097 Free PMC article. - A Mobile Phone App Featuring Cue Exposure Therapy As Aftercare for Alcohol Use Disorders: An Investigator-Blinded Randomized Controlled Trial.
Mellentin AI, Nielsen B, Nielsen AS, Yu F, Mejldal A, Nielsen DG, Stenager E. Mellentin AI, et al. JMIR Mhealth Uhealth. 2019 Aug 16;7(8):e13793. doi: 10.2196/13793. JMIR Mhealth Uhealth. 2019. PMID: 31420960 Free PMC article. Clinical Trial.
References
- Culverhouse R, Bucholz KK, Crowe RR, et al. Long-term stability of alcohol and other substance dependence diagnoses and habitual smoking. JAMA Psychiatry. 2005;62:753–760. - PubMed
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. - PubMed
- Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol. 2001;62(2):211–20. - PubMed
- McLellan AT, McKay JR, Forman R, Cacciola J, Kemp J. Reconsidering the evaluation of addiction treatment: From retrospective follow-up to concurrent recovery monitoring. Addiction. 2005;100:447–458. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous