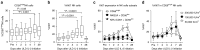Ultra-low dose interleukin-2 promotes immune-modulating function of regulatory T cells and natural killer cells in healthy volunteers - PubMed (original) (raw)
. 2014 Jul;22(7):1388-1395.
doi: 10.1038/mt.2014.50. Epub 2014 Apr 1.
Catherine M Bollard 2, Mattias Carlsten 3, Jan Joseph Melenhorst 4, Angélique Biancotto 5, Ena Wang 6, Jinguo Chen 5, Yuri Kotliarov 5, Foo Cheung 5, Zhi Xie 7, Francesco Marincola 8, Kazushi Tanimoto 9, Minoo Battiwalla 3, Matthew J Olnes 10, Shira Perl 5, Paula Schum 5, Thomas E Hughes 11, Keyvan Keyvanfar 3, Nancy Hensel 3, Pawel Muranski 3, Neal S Young 12, A John Barrett 3
Affiliations
- PMID: 24686272
- PMCID: PMC4089007
- DOI: 10.1038/mt.2014.50
Ultra-low dose interleukin-2 promotes immune-modulating function of regulatory T cells and natural killer cells in healthy volunteers
Sawa Ito et al. Mol Ther. 2014 Jul.
Abstract
Low-dose interleukin-2 (IL-2) expands regulatory T cells (Tregs) and natural killer (NK) cells after stem cell transplantation (SCT) and may reduce graft-versus-host disease (GVHD). We hypothesized that ultra-low dose (ULD) IL-2 could serve as an immune-modulating agent for stem cell donors to prevent GVHD following SCT. However, the safety, dose level, and immune signatures of ULD IL-2 in immune-competent healthy subjects remain unknown. Here, we have characterized the phenotype and function of Tregs and NK cells as well as the gene expression and cytokine profiles of 21 healthy volunteers receiving 50,000 to 200,000 units/m(2)/day IL-2 for 5 days. ULD IL-2 was well tolerated and induced a significant increase in the frequency of Tregs with increased suppressive function. There was a marked expansion of CD56(bright) NK cells with enhanced interferon-γ (IFN-γ) production. Serum cytokine profiling demonstrated increase of IFN-γ induced protein 10 (IP-10). Gene expression analysis revealed significant changes in a highly restricted set of genes, including FOXP3, IL-2RA, and CISH. This is the first study to evaluate global immune-modulating function of ULD IL-2 in healthy subjects and to support the future studies administrating ULD IL-2 to stem cell donors.
Figures
Figure 1
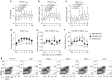
Chronological changes in T regs subsets after ultra-low dose (ULD) IL-2. (a) %FoxP3+CD4 Tregs were significantly increased and peaked at day 4 (mean 3.53 ± 1.17% at day 0 versus 5.68 ± 1.56% at day 4; P < 0.0001). (b,c) Helios+FoxP3+Tregs were significantly increased by day 2 (mean 2.19 ± 1.17% at day 0 versus 3.51 ± 1.03% at day 2; P = 0.004) followed by Helios−FoxP3+Tregs (mean 1.36 ± 0.71% at day 0 versus 1.86 ± 1.16% at day 3; P = 0.01). Both subsets of Tregs significantly expanded, peaking at day 4 and remaining significantly higher than baseline until day 7. (d) Expansion of Helios+FoxP3+Tregs was significantly higher when donors received 100,000 and 200,000 IU/m2 IL-2 compared to 50,000 IU/m2 (P < 0.001 and P = 0.01 at day 4 respectively). (e) Ki67 was significantly induced in Helios+FoxP3+Tregs specifically in 100,000 and 200,000 IU/m2 IL-2 compared to 50,000 IU/m2 (P = 0.03 and P = 0.01 at day 4 respectively). (f) HLA-DR was significantly induced in Helios+FoxP3+Tregs specifically in 100,000 and 200,000 IU/m2 IL-2 compared to 50,000 IU/m2 (P = 0.008 and P = 0.04 at day 4 respectively). (g) The representative flow cytometry data showed expansions of FoxP3+CD4 Tregs in both Helios fractions in healthy volunteer receiving ULD IL-2 (200,000 IU/m2).
Figure 2
Chronological changes in natural killer (NK) cell subsets after ultra-low dose (ULD) IL-2. (a) CD56bright NK cells were significantly increased at day 7 (9.0 ± 4.7% at day 0 versus 12.7 ± 5.8% at day 7; P = 0.001). (b) %Ki67 in NK cell was markedly increased at day 4 (P < 0.0001). (c,d) %Ki67 was significantly induced in CD56bright NK cells compared to CD56dimNKG2A+KIR−, and CD56dimKIR+CD57+ NK populations (26.4 ± 11.4% Ki67+ in CD56bright NK, versus 10.1 ± 7.3% in CD56dimNKG2A+ KIR−NK, 6.1 ± 2.9% CD56dimKIR+CD57+ NK at day 4; P < 0.0001) especially in the cohort of 200,000 IU/m2.
Figure 3
In vitro functions of T regs and natural killer (NK) cells after ultra-low dose (ULD) IL-2. (a) Tcons (CD3+CD4+CD25lowCD127high) equally expressed CD154 through anti-CD3/CD28-coated bead stimulation in pre- and post-IL-2 samples (CD154 expression 52.3 ± 17.5% pre-IL-2 Tcons versus 52.8 ± 17.9% post-IL-2 Tcon; P = 0.86). (b) The suppressive capacity of Tregs on CD154 activation of autologous Tcons was significantly increased in post-IL-2 samples (% suppression 14.0 ± 6.9% pre-IL-2 versus 19.9 ± 8.8% post-IL-2 at Tregs:Tcons ratio 1:1; P = 0.02). (c) Significantly increased interferon (IFN)-γ productions in post-IL-2 NK cells was observed compared to pre-IL-2 NK cells especially in the CD56brightNK cell subset (mean %IFN-γ production 12.0 ± 2.1% pre-IL-2 versus 21.0 ± 4.2% post-IL-2 with IL-12+IL15 and K562 stimulation; P = 0.009). (d) CD107a expression was not significantly different pre- versus post-IL-2 injections (mean %CD107a expression in CD56dimNK cells, 9.6 ± 3.6% pre-IL-2 versus 9.3 ± 3.7% post-IL-2 with IL-12+IL15 and K562 stimulation; P = 0.75).
Figure 4
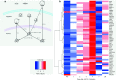
Genes differentially expressed by ultra-low dose (ULD) IL-2 in healthy volunteers. (a) Functional annotation of ULD IL-2 related genes: functional relationships are annotated to the genes differentially expressed on day 4 after ULD IL-2 using IPA Ingenuity Path Designer. (b) Time-course analysis of differentially expressed genes after ULD IL-2: hierarchical clustering of genes differentially expressed after ULD IL-2 is shown in a heatmap. Days are represented in the columns and probe sets in the rows.
Similar articles
- Low-dose IL-2 selectively activates subsets of CD4+ Tregs and NK cells.
Hirakawa M, Matos TR, Liu H, Koreth J, Kim HT, Paul NE, Murase K, Whangbo J, Alho AC, Nikiforow S, Cutler C, Ho VT, Armand P, Alyea EP, Antin JH, Blazar BR, Lacerda JF, Soiffer RJ, Ritz J. Hirakawa M, et al. JCI Insight. 2016 Nov 3;1(18):e89278. doi: 10.1172/jci.insight.89278. JCI Insight. 2016. PMID: 27812545 Free PMC article. - Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity.
Kennedy-Nasser AA, Ku S, Castillo-Caro P, Hazrat Y, Wu MF, Liu H, Melenhorst J, Barrett AJ, Ito S, Foster A, Savoldo B, Yvon E, Carrum G, Ramos CA, Krance RA, Leung K, Heslop HE, Brenner MK, Bollard CM. Kennedy-Nasser AA, et al. Clin Cancer Res. 2014 Apr 15;20(8):2215-25. doi: 10.1158/1078-0432.CCR-13-3205. Epub 2014 Feb 26. Clin Cancer Res. 2014. PMID: 24573552 Free PMC article. Clinical Trial. - Thymoglobulin, interferon-γ and interleukin-2 efficiently expand cytokine-induced killer (CIK) cells in clinical-grade cultures.
Bonanno G, Iudicone P, Mariotti A, Procoli A, Pandolfi A, Fioravanti D, Corallo M, Perillo A, Scambia G, Pierelli L, Rutella S. Bonanno G, et al. J Transl Med. 2010 Dec 7;8:129. doi: 10.1186/1479-5876-8-129. J Transl Med. 2010. PMID: 21138560 Free PMC article. - Functional analyses of cord blood natural killer cells and T cells: a distinctive interleukin-18 response.
Nomura A, Takada H, Jin CH, Tanaka T, Ohga S, Hara T. Nomura A, et al. Exp Hematol. 2001 Oct;29(10):1169-76. doi: 10.1016/s0301-472x(01)00689-0. Exp Hematol. 2001. PMID: 11602318 Review. - The regulation and biological activity of interleukin 12.
Lee SM, Suen Y, Qian J, Knoppel E, Cairo MS. Lee SM, et al. Leuk Lymphoma. 1998 May;29(5-6):427-38. doi: 10.3109/10428199809050903. Leuk Lymphoma. 1998. PMID: 9643557 Review.
Cited by
- Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: the force awakens.
Childs RW, Carlsten M. Childs RW, et al. Nat Rev Drug Discov. 2015 Jul;14(7):487-98. doi: 10.1038/nrd4506. Epub 2015 May 22. Nat Rev Drug Discov. 2015. PMID: 26000725 Review. - Low-dose interleukin-2-loaded nanoparticle effect on NK and T-reg cell expression in experimentally induced type 1 diabetes mellitus.
Aboelnazar S, Ghoneim H, Moaaz M, Bahgat ET, Shalaby T. Aboelnazar S, et al. Prz Gastroenterol. 2021;16(1):67-82. doi: 10.5114/pg.2021.104737. Epub 2021 Mar 26. Prz Gastroenterol. 2021. PMID: 33986891 Free PMC article. - Low Dose of IL-2 Normalizes Hypertension and Mitochondrial Function in the RUPP Rat Model of Placental Ischemia.
Deer E, Amaral LM, Campbell N, Fitzgerald S, Herrock O, Ibrahim T, LaMarca B. Deer E, et al. Cells. 2021 Oct 19;10(10):2797. doi: 10.3390/cells10102797. Cells. 2021. PMID: 34685775 Free PMC article. - The Biological Role and Therapeutic Potential of NK Cells in Hematological and Solid Tumors.
Velichinskii RA, Streltsova MA, Kust SA, Sapozhnikov AM, Kovalenko EI. Velichinskii RA, et al. Int J Mol Sci. 2021 Oct 21;22(21):11385. doi: 10.3390/ijms222111385. Int J Mol Sci. 2021. PMID: 34768814 Free PMC article. Review. - Next Generation Natural Killer Cells for Cancer Immunotherapy.
Rossi F, Fredericks N, Snowden A, Allegrezza MJ, Moreno-Nieves UY. Rossi F, et al. Front Immunol. 2022 Jun 2;13:886429. doi: 10.3389/fimmu.2022.886429. eCollection 2022. Front Immunol. 2022. PMID: 35720306 Free PMC article. Review.
References
- Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. - PubMed
- Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169–1176. - PubMed
- Macdonald D, Jiang YZ, Gordon AA, Mahendra P, Oskam R, Palmer PA, et al. Recombinant interleukin 2 for acute myeloid leukaemia in first complete remission: a pilot study. Leuk Res. 1990;14:967–973. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials