Direct visualization of HIV-enhancing endogenous amyloid fibrils in human semen - PubMed (original) (raw)
Onofrio Zirafi 1, Janis A Müller 1, Nathallie L Sandi-Monroy 2, Jay K Yadav 3, Christoph Meier 4, Tanja Weil 4, Nadia R Roan 5, Warner C Greene 6, Paul Walther 7, K Peter R Nilsson 8, Per Hammarström 8, Ronald Wetzel 9, Christopher D Pilcher 10, Friedrich Gagsteiger 11, Marcus Fändrich 3, Frank Kirchhoff 1, Jan Münch 1
Affiliations
- PMID: 24691351
- PMCID: PMC4129123
- DOI: 10.1038/ncomms4508
Direct visualization of HIV-enhancing endogenous amyloid fibrils in human semen
Shariq M Usmani et al. Nat Commun. 2014.
Abstract
Naturally occurring fragments of the abundant semen proteins prostatic acid phosphatase (PAP) and semenogelins form amyloid fibrils in vitro. These fibrils boost HIV infection and may play a key role in the spread of the AIDS pandemic. However, the presence of amyloid fibrils in semen remained to be demonstrated. Here, we use state of the art confocal and electron microscopy techniques for direct imaging of amyloid fibrils in human ejaculates. We detect amyloid aggregates in all semen samples and find that they partially consist of PAP fragments, interact with HIV particles and increase viral infectivity. Our results establish semen as a body fluid that naturally contains amyloid fibrils that are exploited by HIV to promote its sexual transmission.
Figures
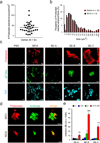
Figure 1. Detection of endogenous amyloid in human semen with fluorescent dyes and fibril-specific antibodies
(a) Number of Proteostat-stained structures in semen from individual donors. Liquefied ejaculates were stained with the amyloid-specific Proteostat dye at room temperature for 15- 20 min and imaged using a LSM710 confocal microscope. (b) Size distribution of endogenous amyloid in semen, and in vitro generated SEVI fibrils in buffer. (c) Confocal microscopy images of in vitro generated SEVI fibrils and of semen samples (SE-A to SE-C) stained with amyloid-specific probes Proteostat, pFTAA, and ThT. Scale bar = 5 µm. PBS, negative control. (d) Detection of amyloid fibrils in semen using amyloid-specific conformational antibodies. Semen was treated with WO1 and WO2 antibodies, and then amyloid/antibody complexes were pelleted, washed, and detected using Alexa488-coupled appropriate secondary antibody (green), and counterstained with Proteostat dye (red). Scale bar = 5 µm. (e) Effect of semen samples A-C on HIV infection. R5-tropic HIV-1 was exposed to indicated concentrations of semen (%), and used to infect TZM-bl cells. After 2 h, semen-virion mixtures were replaced with fresh medium. HIV-1 infection rates were measured three days later by quantifying β-galactosidase activities in cell lysates. Values shown are average values derived from triplicate infection ± standard deviation. The numbers above the columns give the n-fold enhancement of infection observed after treatment with 10 % semen relative to infection with mock-treated virus.
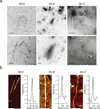
Figure 2. Ultrastructural analyses of endogenous amyloid in semen
(a) Transmission electron micrographs of semen. In vitro generated SEVI fibrils or semen samples SE-B and SE-C were dropped onto EM grids which were then washed with PBS after 5 min, and then negatively stained with 2 % uranyl acetate. All samples were immediately analyzed. Scale bars indicate 500 nm for top panel images and 200 nm for bottom panel images. (b) Atomic force micrographs of synthetic SEVI fibrils and endogenous fibrils in semen samples SE-B and Se-C, which were placed on silicon substrates, washed with H2O, and imaged in air. Scale bar = 200 nm. The height profiles represent the averages of multiple cross-sections along the fibrils
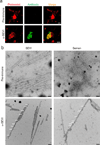
Figure 3. Endogenous amyloids partially consists of SEVI
(a) Semen was treated with pre-immune (top) or anti-SEVI antiserum (α-SEVI, bottom). The amyloid/antibody complexes were pelleted, washed, and incubated with an Alexa488-coupled secondary antibody (green), and counterstained with Proteostat dye (red). Scale bar = 5 µm. (b) Immunogold-labeling of endogenous SEVI fibrils in semen. Transmission electron micrographs of semen treated with a pre-immune serum or an anti-SEVI antiserum as primary antibodies, and gold conjugated anti-rabbit secondary antibody. Scale bar = 100 nm. White arrows indicate gold particles bound to amyloid fibrils.
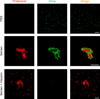
Figure 4. Seminal amyloid interacts with retroviral particles
EYFP-labeled virions (green) were mixed with PBS or semen that was stained with Proteostat dye (red). Images were acquired 15 min later on a laser scanning confocal microscope. Single plane images from the centre of the Z-stack are shown. Treatment of semen with heparin (100 µg/ml) abrogates the ability of seminal amyloid to bind viral particles. Scale bar = 5 µm. Also see Supplementary Movie 1.
Similar articles
- Liquefaction of semen generates and later degrades a conserved semenogelin peptide that enhances HIV infection.
Roan NR, Liu H, Usmani SM, Neidleman J, Müller JA, Avila-Herrera A, Gawanbacht A, Zirafi O, Chu S, Dong M, Kumar ST, Smith JF, Pollard KS, Fändrich M, Kirchhoff F, Münch J, Witkowska HE, Greene WC. Roan NR, et al. J Virol. 2014 Jul;88(13):7221-34. doi: 10.1128/JVI.00269-14. Epub 2014 Apr 16. J Virol. 2014. PMID: 24741080 Free PMC article. - Peptides released by physiological cleavage of semen coagulum proteins form amyloids that enhance HIV infection.
Roan NR, Müller JA, Liu H, Chu S, Arnold F, Stürzel CM, Walther P, Dong M, Witkowska HE, Kirchhoff F, Münch J, Greene WC. Roan NR, et al. Cell Host Microbe. 2011 Dec 15;10(6):541-50. doi: 10.1016/j.chom.2011.10.010. Cell Host Microbe. 2011. PMID: 22177559 Free PMC article. - Semen-derived amyloid fibrils drastically enhance HIV infection.
Münch J, Rücker E, Ständker L, Adermann K, Goffinet C, Schindler M, Wildum S, Chinnadurai R, Rajan D, Specht A, Giménez-Gallego G, Sánchez PC, Fowler DM, Koulov A, Kelly JW, Mothes W, Grivel JC, Margolis L, Keppler OT, Forssmann WG, Kirchhoff F. Münch J, et al. Cell. 2007 Dec 14;131(6):1059-71. doi: 10.1016/j.cell.2007.10.014. Cell. 2007. PMID: 18083097 - Semen-derived amyloidogenic peptides-Key players of HIV infection.
Lee YH, Ramamoorthy A. Lee YH, et al. Protein Sci. 2018 Jul;27(7):1151-1165. doi: 10.1002/pro.3395. Epub 2018 Mar 14. Protein Sci. 2018. PMID: 29493036 Free PMC article. Review. - Role of semen in HIV-1 transmission: inhibitor or facilitator?
Doncel GF, Joseph T, Thurman AR. Doncel GF, et al. Am J Reprod Immunol. 2011 Mar;65(3):292-301. doi: 10.1111/j.1600-0897.2010.00931.x. Epub 2010 Nov 19. Am J Reprod Immunol. 2011. PMID: 21087339 Review.
Cited by
- Collective Viral Spread Mediated by Virion Aggregates Promotes the Evolution of Defective Interfering Particles.
Andreu-Moreno I, Sanjuán R. Andreu-Moreno I, et al. mBio. 2020 Jan 7;11(1):e02156-19. doi: 10.1128/mBio.02156-19. mBio. 2020. PMID: 31911487 Free PMC article. - Sulfonated Compounds Bind with Prostatic Acid Phosphatase (PAP248-286) to Inhibit the Formation of Amyloid Fibrils.
Zhang T, Yang H, Yang Z, Tan S, Jin J, Liu S, Zhang J. Zhang T, et al. ChemistryOpen. 2018 Jun 11;7(6):447-456. doi: 10.1002/open.201800041. eCollection 2018 Jun. ChemistryOpen. 2018. PMID: 29928568 Free PMC article. - Rapid Formation of Peptide/Lipid Coaggregates by the Amyloidogenic Seminal Peptide PAP248-286.
Vane EW, He S, Maibaum L, Nath A. Vane EW, et al. Biophys J. 2020 Sep 1;119(5):924-938. doi: 10.1016/j.bpj.2020.07.029. Epub 2020 Aug 6. Biophys J. 2020. PMID: 32814060 Free PMC article. - Arabidopsis thaliana Phytocystatin 6 Forms Functional Oligomer and Amyloid Fibril States.
Santos NP, Brandstetter H, Dall E. Santos NP, et al. Biochemistry. 2023 Dec 5;62(23):3420-3429. doi: 10.1021/acs.biochem.3c00530. Epub 2023 Nov 21. Biochemistry. 2023. PMID: 37989209 Free PMC article. - Stereochemical identification of glucans by oligothiophenes enables cellulose anatomical mapping in plant tissues.
Choong FX, Bäck M, Schulz A, Nilsson KPR, Edlund U, Richter-Dahlfors A. Choong FX, et al. Sci Rep. 2018 Feb 15;8(1):3108. doi: 10.1038/s41598-018-21466-y. Sci Rep. 2018. PMID: 29449697 Free PMC article.
References
- Lederman MM, Offord RE, Hartley O. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat Rev Immunol. 2006;6:371–382. - PubMed
- Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. - PubMed
- Allen RD, Roberts TK. The relationship between the immunosuppressive and cytotoxic effects of human seminal plasma. Am J Reprod Immunol Microbiol. 1987;11:59–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AI071713/AI/NIAID NIH HHS/United States
- R01 NS046356/NS/NINDS NIH HHS/United States
- K12 DK083021/DK/NIDDK NIH HHS/United States
- P01 AI083050/AI/NIAID NIH HHS/United States
- 1P01 AI083050-01/AI/NIAID NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- K99 AI104262/AI/NIAID NIH HHS/United States
- R01 HD074511/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials