Biological 2-input decoder circuit in human cells - PubMed (original) (raw)
. 2014 Aug 15;3(8):627-33.
doi: 10.1021/sb4001596. Epub 2014 Apr 11.
Affiliations
- PMID: 24694115
- PMCID: PMC4165469
- DOI: 10.1021/sb4001596
Biological 2-input decoder circuit in human cells
Michael Guinn et al. ACS Synth Biol. 2014.
Abstract
Decoders are combinational circuits that convert information from n inputs to a maximum of 2(n) outputs. This operation is of major importance in computing systems yet it is vastly underexplored in synthetic biology. Here, we present a synthetic gene network architecture that operates as a biological decoder in human cells, converting 2 inputs to 4 outputs. As a proof-of-principle, we use small molecules to emulate the two inputs and fluorescent reporters as the corresponding four outputs. The experiments are performed using transient transfections in human kidney embryonic cells and the characterization by fluorescence microscopy and flow cytometry. We show a clear separation between the ON and OFF mean fluorescent intensity states. Additionally, we adopt the integrated mean fluorescence intensity for the characterization of the circuit and show that this metric is more robust to transfection conditions when compared to the mean fluorescent intensity. To conclude, we present the first implementation of a genetic decoder. This combinational system can be valuable toward engineering higher-order circuits as well as accommodate a multiplexed interface with endogenous cellular functions.
Figures
Figure 1
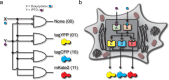
(a) Boolean logic illustration of a biological decoder with chemical inputs and fluorescence proteins as the outputs of the genetic circuit. (b) Node and edge schematic of the decoder circuit within a cell. The system consists of five distinct nodes combined into two distinct layers which interact through activation and inhibition edges. Nodes A and B comprise the “regulating” nodes, while nodes C, D, and E comprise the “regulated” nodes.
Figure 2
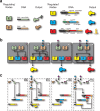
(a) Illustration showing the system nodes, the DNA architecture of each node, and the outputs of each node of the decoder. There are two layers of the decoder architecture: a “regulating” layer and a “regulated” layer. The “regulating” layer consists of two nodes: node A and node B. Both node A and B constitutively produce gene products which are the transcription factors rtTA and LacI-KRAB. Node A responds to the input IPTG by becoming inactivated. Node B responds to the input DOX by becoming activated. The “regulated” nodes consist of three nodes: node C, D, and E. Each node consists of combinations of promoters, transcription factors, operator sites, fluorescence proteins, synthetic microRNAs, and degradation tags. (b) Node and edge diagram for each state of the decoder, showing active edges for each individual state. In the “00” state (IPTG:0, DOX:0), node A is active and node B is inactive. Node A inhibits nodes D and E, while node B cannot activate nodes C and E. This results in the nonproduct case of the decoder. In the “01” state (IPTG:0, DOX:0), node A is active and node B is active. Node A inhibits nodes D and E, while node B activates nodes C and E. This results in the production of tagYFP as the decoder output. In the “10” state (IPTG:1, DOX:0), node A is inactive and node B is inactive. Node A is prevented from inhibiting nodes C and E, while node B cannot activate nodes C and E. This results in the production of tagCFP as the decoder output. In the “11” state (IPTG:1, DOX:1), node A is inactive and node B is active. Node A is prevented from inhibiting nodes C and E, while node B activates nodes C and E. This results in mRNA output of all three nodes (C, D, and E). However, a feedback mechanism is incorporated into node E which prevents the translation of the outputs from nodes C and D. This results in mKate2 as the decoder output. (c) Biological interactions of nodal components within the decoder architecture for each Boolean state. The four decoder cases are shown to illustrate the interactions the inputs have with the effector proteins, the interactions the effector proteins have with the DNA components, and the interactions between the feedback mechanism and its accompanying targets.
Figure 3
(a) Histograms of kidney cells transfected with the decoder circuit (50 ng per node) for the four states of the decoder. Each row shows histograms for each color of each state, while each column shows each state. The ON states (“01”for tagYFP, “10” for tagCFP, and “11” mKate2) for each state of the decoder show significant increase from all OFF states. Additionally, in each histogram in the upper right-hand corner is the number of cells above the 102au threshold. (b) Fluorescence microscopy of the four outputs. There are five columns: a bright field showing the live cells, a tagYFP, tagCFP, and mKate2 field, and last an overlap of the three fluorescence fields. Each row is a different state of the decoder. Each field is normalized to the same intensity, so that images from the same column can be compared with each other. (c) The normalized background subtracted mean fluorescence intensity of single cells. The MFI corresponds to the data shown in panel a. Each state of the decoder shows the correct ON signal in the appropriate case, with leakage significantly lower in all OFF states. (d) Flow cytometry scatter plots showing the four states of the system. Each column shows a different fluorescent output on the _y_-axis and each row shows a different state of the circuit. In each scatter plot, in the upper right-hand corner is the frequency of cells that fall above the background fluorescence gate (102 au). The black dots on the scatter plots are single cells that fall below the background fluorescence threshold, while the colored dots are single cells that fall above this threshold. The bar graph on the side of the FACs scatter plots shows the integrated mean fluorescence intensity (iMFI) for each state of the decoder. This correlates with the MFI quantity, showing a 4–5 fold change between all ON and OFF states.
Similar articles
- Monitoring Promoter Activity by Flow Cytometry.
Taher TEI. Taher TEI. Methods Mol Biol. 2017;1651:65-73. doi: 10.1007/978-1-4939-7223-4_6. Methods Mol Biol. 2017. PMID: 28801900 - Precise determination of input-output mapping for multimodal gene circuits using data from transient transfection.
Stelzer C, Benenson Y. Stelzer C, et al. PLoS Comput Biol. 2020 Nov 30;16(11):e1008389. doi: 10.1371/journal.pcbi.1008389. eCollection 2020 Nov. PLoS Comput Biol. 2020. PMID: 33253149 Free PMC article. - A synthetic gene circuit for measuring autoregulatory feedback control.
Schikora-Tamarit MÀ, Toscano-Ochoa C, Domingo Espinós J, Espinar L, Carey LB. Schikora-Tamarit MÀ, et al. Integr Biol (Camb). 2016 Apr 18;8(4):546-55. doi: 10.1039/c5ib00230c. Epub 2016 Jan 5. Integr Biol (Camb). 2016. PMID: 26728081 - Quantitative modeling of the interplay between synthetic gene circuits and host physiology: experiments, results, and prospects.
Sánchez-Osorio I, Hernández-Martínez CA, Martínez-Antonio A. Sánchez-Osorio I, et al. Curr Opin Microbiol. 2020 Jun;55:48-56. doi: 10.1016/j.mib.2020.02.008. Epub 2020 Mar 25. Curr Opin Microbiol. 2020. PMID: 32220744 Review. - The design of synthetic gene circuits in plants: new components, old challenges.
Vazquez-Vilar M, Selma S, Orzaez D. Vazquez-Vilar M, et al. J Exp Bot. 2023 Jul 18;74(13):3791-3805. doi: 10.1093/jxb/erad167. J Exp Bot. 2023. PMID: 37204924 Free PMC article. Review.
Cited by
- Large-scale design of robust genetic circuits with multiple inputs and outputs for mammalian cells.
Weinberg BH, Pham NTH, Caraballo LD, Lozanoski T, Engel A, Bhatia S, Wong WW. Weinberg BH, et al. Nat Biotechnol. 2017 May;35(5):453-462. doi: 10.1038/nbt.3805. Epub 2017 Mar 27. Nat Biotechnol. 2017. PMID: 28346402 Free PMC article. - Cell morphology-based machine learning models for human cell state classification.
Li Y, Nowak CM, Pham U, Nguyen K, Bleris L. Li Y, et al. NPJ Syst Biol Appl. 2021 May 26;7(1):23. doi: 10.1038/s41540-021-00180-y. NPJ Syst Biol Appl. 2021. PMID: 34039992 Free PMC article. - Towards control of cellular decision-making networks in the epithelial-to-mesenchymal transition.
Gómez Tejeda Zañudo J, Guinn MT, Farquhar K, Szenk M, Steinway SN, Balázsi G, Albert R. Gómez Tejeda Zañudo J, et al. Phys Biol. 2019 Mar 7;16(3):031002. doi: 10.1088/1478-3975/aaffa1. Phys Biol. 2019. PMID: 30654341 Free PMC article. Review. - Perfluorooctanoic Acid and Its Short-Chain Substitutes Induce Cytotoxic and Prooxidative Changes in Human Peripheral Blood Mononuclear Cells: A Comparative Study.
Kaczmarska I, Mokra K, Michałowicz J. Kaczmarska I, et al. Int J Mol Sci. 2025 Jun 5;26(11):5408. doi: 10.3390/ijms26115408. Int J Mol Sci. 2025. PMID: 40508216 Free PMC article. - Mapping the operational landscape of microRNAs in synthetic gene circuits.
Quarton T, Ehrhardt K, Lee J, Kannan S, Li Y, Ma L, Bleris L. Quarton T, et al. NPJ Syst Biol Appl. 2018 Jan 11;4:6. doi: 10.1038/s41540-017-0043-y. eCollection 2018. NPJ Syst Biol Appl. 2018. PMID: 29354284 Free PMC article.
References
- Benenson Y. (2012) Biomolecular computing systems: Principles, progress and potential. Nat. Rev. Genet. 13, 455–468. - PubMed
- Ro D.; Paradise E. M.; Ouellet M.; Fisher K. J.; Newman K. L.; Ndungu J. M.; Ho K. A.; Eachus R. A.; Ham T. S.; Kirby J.; Chang M. C. Y.; Withers S. T.; Shiba Y.; Sarpong R.; Keasling J. D. (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440, 940–943. - PubMed
- Bokinsky G.; Peralta-Yahya P. P.; George A.; Holmes B. M.; Steen E. J.; Dietrich J.; Soon Lee T.; Tullman-Ercek D.; Voigt C. A.; Simmons B. A.; Keasling J. D. (2011) Synthesis of three advanced biofuels from ionic liquid-pretreated switchgrass using engineered Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 108, 19949–19954. - PMC - PubMed
- Xie Z.; Wroblewska L.; Prochazka L.; Weiss R.; Benenson Y. (2011) Multi-input RNAi-based logic circuit for identification of specific cancer cells. Science 333, 1307–1311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous