Macrophages: a double-edged sword in experimental autoimmune encephalomyelitis - PubMed (original) (raw)
Review
Macrophages: a double-edged sword in experimental autoimmune encephalomyelitis
Zhilong Jiang et al. Immunol Lett. 2014 Jul.
Abstract
Multiple sclerosis (MS) is a debilitating neurological disorder of the central nervous system (CNS), characterized by activation and infiltration of leukocytes and dendritic cells into the CNS. In the initial phase of MS and its animal model, experimental autoimmune encephalomyelitis (EAE), peripheral macrophages infiltrate into the CNS, where, together with residential microglia, they participate in the induction and development of disease. During the early phase, microglia/macrophages are immediately activated to become classically activated macrophages (M1 cells), release pro-inflammatory cytokines and damage CNS tissue. During the later phase, microglia/macrophages in the inflamed CNS are less activated, present as alternatively activated macrophage phenotype (M2 cells), releasing anti-inflammatory cytokines, accompanied by inflammation resolution and tissue repair. The balance between activation and polarization of M1 cells and M2 cells in the CNS is important for disease progression. Pro-inflammatory IFN-γ and IL-12 drive M1 cell polarization, while IL-4 and IL-13 drive M2 cell polarization. Given that polarized macrophages are reversible in a well-defined cytokine environment, macrophage phenotypes in the CNS can be modulated by molecular intervention. This review summarizes the detrimental and beneficial roles of microglia and macrophages in the CNS, with an emphasis on the role of M2 cells in EAE and MS patients.
Keywords: Cytokines; Experimental autoimmune encephalomyelitis; Macrophages; Multiple sclerosis.
Copyright © 2014 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors declare no conflicts of interest.
Figures
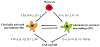
Fig. 1.
Scheme for immune pathways leading to activation of macrophage into two subtypes. M1 cells dominantly express TNF-α, IL-1β, IL-12, NO, and induce CNS inflammation induction and tissue damage. M2 cells have specific maker CD206 (MR), dominantly express IL-4, IL-10, IL-13, TGF-β, and induce CNS inflammation resolution and tissue repair.
Similar articles
- The roles of macrophages and microglia in multiple sclerosis and experimental autoimmune encephalomyelitis.
Chu F, Shi M, Zheng C, Shen D, Zhu J, Zheng X, Cui L. Chu F, et al. J Neuroimmunol. 2018 May 15;318:1-7. doi: 10.1016/j.jneuroim.2018.02.015. Epub 2018 Feb 27. J Neuroimmunol. 2018. PMID: 29606295 Review. - A novel PADRE-Kv1.3 vaccine effectively induces therapeutic antibodies and ameliorates experimental autoimmune encephalomyelitis in rats.
Fan C, Long R, You Y, Wang J, Yang X, Huang S, Sheng Y, Peng X, Liu H, Wang Z, Liu K. Fan C, et al. Clin Immunol. 2018 Aug;193:98-109. doi: 10.1016/j.clim.2018.02.012. Epub 2018 Feb 27. Clin Immunol. 2018. PMID: 29496642 - Cyclic AMP Pathway Suppress Autoimmune Neuroinflammation by Inhibiting Functions of Encephalitogenic CD4 T Cells and Enhancing M2 Macrophage Polarization at the Site of Inflammation.
Veremeyko T, Yung AWY, Dukhinova M, Kuznetsova IS, Pomytkin I, Lyundup A, Strekalova T, Barteneva NS, Ponomarev ED. Veremeyko T, et al. Front Immunol. 2018 Jan 25;9:50. doi: 10.3389/fimmu.2018.00050. eCollection 2018. Front Immunol. 2018. PMID: 29422898 Free PMC article. - Sex-Specific Effects of Microglia-Like Cell Engraftment during Experimental Autoimmune Encephalomyelitis.
Han J, Zhu K, Zhou K, Hakim R, Sankavaram SR, Blomgren K, Lund H, Zhang XM, Harris RA. Han J, et al. Int J Mol Sci. 2020 Sep 17;21(18):6824. doi: 10.3390/ijms21186824. Int J Mol Sci. 2020. PMID: 32957621 Free PMC article. - The benefits and detriments of macrophages/microglia in models of multiple sclerosis.
Rawji KS, Yong VW. Rawji KS, et al. Clin Dev Immunol. 2013;2013:948976. doi: 10.1155/2013/948976. Epub 2013 Jun 12. Clin Dev Immunol. 2013. PMID: 23840244 Free PMC article. Review.
Cited by
- Estrogen induces multiple regulatory B cell subtypes and promotes M2 microglia and neuroprotection during experimental autoimmune encephalomyelitis.
Benedek G, Zhang J, Bodhankar S, Nguyen H, Kent G, Jordan K, Manning D, Vandenbark AA, Offner H. Benedek G, et al. J Neuroimmunol. 2016 Apr 15;293:45-53. doi: 10.1016/j.jneuroim.2016.02.009. Epub 2016 Feb 13. J Neuroimmunol. 2016. PMID: 27049561 Free PMC article. - Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages.
Dey A, Allen J, Hankey-Giblin PA. Dey A, et al. Front Immunol. 2015 Jan 22;5:683. doi: 10.3389/fimmu.2014.00683. eCollection 2014. Front Immunol. 2015. PMID: 25657646 Free PMC article. Review. - Loss of Allograft Inflammatory Factor-1 Ameliorates Experimental Autoimmune Encephalomyelitis by Limiting Encephalitogenic CD4 T-Cell Expansion.
Chinnasamy P, Lutz SE, Riascos-Bernal DF, Jeganathan V, Casimiro I, Brosnan CF, Sibinga NE. Chinnasamy P, et al. Mol Med. 2015 Jan 6;21(1):233-41. doi: 10.2119/molmed.2014.00264. Mol Med. 2015. PMID: 25569805 Free PMC article. - Mechanisms of M2 Macrophage-Derived Exosomal Long Non-coding RNA PVT1 in Regulating Th17 Cell Response in Experimental Autoimmune Encephalomyelitisa.
Wu L, Xia J, Li D, Kang Y, Fang W, Huang P. Wu L, et al. Front Immunol. 2020 Sep 4;11:1934. doi: 10.3389/fimmu.2020.01934. eCollection 2020. Front Immunol. 2020. PMID: 33013847 Free PMC article. Retracted. - The Emerging Role of Microglial Hv1 as a Target for Immunomodulation in Myelin Repair.
Tang Y, Wu X, Li J, Li Y, Xu X, Li G, Zhang P, Qin C, Wu LJ, Tang Z, Tian DS. Tang Y, et al. Aging Dis. 2024 May 7;15(3):1176-1203. doi: 10.14336/AD.2023.1107. Aging Dis. 2024. PMID: 38029392 Free PMC article. Review.
References
- Hickey WF. The pathology of multiple sclerosis: a historical perspective. J Neuroimmunol 1999;98:37–44. - PubMed
- Banwell B, Bar-Or A, Arnold DL, Sadovnick D, Narayanan S, McGowan M, et al. Clinical, environmental, and genetic determinants of multiple sclerosis in children with acute demyelination: a prospective national cohort study. Lancet Neurol 2011;10:436–45. - PubMed
- Lassmann H, Bruck W, Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol Med 2001;7:115–21. - PubMed
- Almolda B, Gonzalez B, Castellano B. Antigen presentation in EAE: role of microglia, macrophages and dendritic cells. Front Biosci 2011;16: 1157–71. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources